If you are interested in products and services related to the research phase in this field, please contact for further inquiries.
Pediatric medical devices are essential tools in the diagnosis, treatment, and management of various medical conditions in children. However, the development and regulation of these devices face unique challenges compared to their adult counterparts. This article aims to provide an in-depth analysis of the current state of pediatric medical devices, the barriers that hinder their development, and the opportunities that lie ahead.
The Current Landscape of Pediatric Medical Devices
The development and regulation of medical devices in the United States are overseen by the Food and Drug Administration (FDA). Devices are classified into three categories based on their risk level: Class I (low risk), Class II (moderate risk), and Class III (high risk). Each category has specific regulatory requirements that must be met before a device can be marketed.
- Class I Devices: These low-risk devices, such as thermometers and surgical tools, make up approximately 47% of all medical devices. Most Class I devices are exempt from review and only need to follow general controls, which include regulations on promotion, labeling, and report management.
- Class II Devices: Moderate-risk devices like powered wheelchairs and surgical clips account for about 43% of medical devices. These devices require both general and special controls, which are device-specific standards intended to ensure safety, performance, and effectiveness.
- Class III Devices: High-risk devices, such as pacemakers and ventilators, constitute 10% of all devices. These devices undergo the most rigorous regulatory scrutiny and must demonstrate safety and efficacy through clinical trials before approval.
Despite the regulatory framework, pediatric medical devices lag behind adult devices in terms of availability and technological sophistication. The FDA reported in 2018 that novel pediatric devices numbered only a quarter of those designed, evaluated, and approved for adults. This disparity is partly due to the unique challenges associated with pediatric device development.
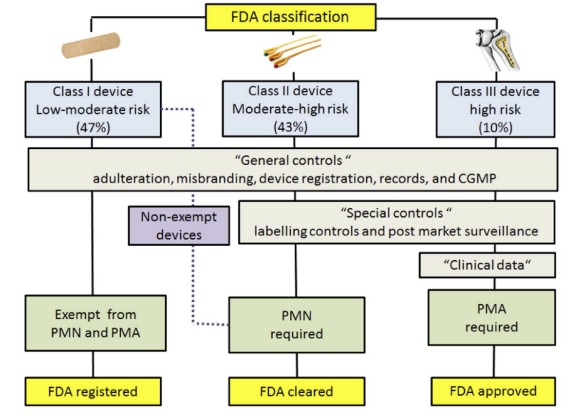 Fig.1 The FDA classifies devices based on a three-tier, risk-based system. (Espinoza J., et al., 2022)
Fig.1 The FDA classifies devices based on a three-tier, risk-based system. (Espinoza J., et al., 2022)
Clinical Considerations
Children are not simply smaller versions of adults; they have distinct physiological, neurodevelopmental, and epidemiological characteristics. These differences necessitate specialized medical devices that can accommodate their unique needs.
Physiological Differences
Children's organ systems, such as the cardiovascular, respiratory, and renal systems, change significantly from childhood to adulthood. For example, the smaller size and progressive growth of children pose engineering challenges for medical device developers. Devices that require active participation from the patient, such as dry powder inhalers, may not be suitable for young children.
Neurodevelopmental Considerations
Devices that require interaction must consider the cognitive capacities of children. For instance, young children may struggle to use certain medical devices effectively, which can impact the device's efficacy and safety.
Activities of Daily Living
Children's typical activities can cause unexpected device performance issues. For example, electronic failures of cochlear implants in children have been reported due to static electricity discharge when sliding down plastic slides in winter snowsuits.
Technical Considerations
The development of pediatric medical devices involves overcoming significant technical challenges.
- Miniaturization: Some technologies cannot be miniaturized or are less effective when made smaller. In some cases, completely different technologies must be used to achieve the desired functionality in a smaller form factor.
- Growth Accommodation: Devices that can expand with the child's growth are difficult to engineer. For example, children with congenital heart disease treated with fixed-size devices like prosthetic valves may require multiple surgeries over their lifetime.
- Material Longevity: Implantable devices in children must last decades, compared to similar devices in adults. This introduces concerns about biocompatibility, leaching, and degradation of materials over time.
Regulatory and Ethical Considerations
The regulatory and ethical landscape for pediatric medical devices is complex and multifaceted.
- Clinical Trials: Conducting clinical trials with small, heterogeneous pediatric populations is challenging. Recruiting patients can be difficult, especially if parents are hesitant to enroll their children in research studies. Additionally, study blinding and the use of sham devices may not be feasible or ethical in pediatric populations.
- Local Regulatory Expertise: Medical device expertise may be lacking in local Institutional Review Boards (IRBs), which can delay approvals. The complexity of medical device regulation has increased over the past several decades, adding to the regulatory uncertainty and time to clearance or approval.
- Labeling and Reimbursement: The FDA does not have specific guidance for pediatric device labeling, leading to confusion and heterogeneity in the labeling of pediatric devices. This can affect coverage and reimbursement decisions, as well as the promotion and marketing of the devices.
Financial Considerations
The financial landscape for pediatric medical devices is also challenging.
Market Size: The pediatric market is smaller than the adult market, making it less attractive for companies to invest in pediatric-specific solutions. The regulatory and technical challenges associated with pediatric devices further exacerbate the financial burden.
Reimbursement Rates: Pediatric care is often reimbursed at a lower rate than adult care, reducing the financial incentives for companies to develop pediatric devices. Additionally, the lack of national coverage decisions for Medicaid, which covers over 50% of children, results in a highly variable reimbursement landscape.
Funding and Infrastructure: There is a lack of funding and infrastructure for the design, evaluation, and validation of pediatric medical devices. This limits the number of innovative solutions that can be brought to market.
Initiatives to Support Pediatric Medical Device Innovation
Several initiatives have been launched to address the challenges in pediatric medical device development and promote innovation in this area.
Office of Orphan Product Development (OOPD)
The OOPD evaluates and assists in the development of devices for rare diseases or conditions. It offers grants and vouchers to offset the financial risks associated with targeting smaller markets. For example, the Orphan Products Grants Program provides funding for the development of devices that benefit patients with rare conditions.
Pediatric Device Consortia (PDC)
Established in 2007, the PDC program funds consortia to promote the development, production, and distribution of pediatric medical devices. To date, the program has supported over 1,000 medical device projects. The current PDC cycle (2018-2023) includes five consortia, each led by a different institution, such as Children’s National Hospital and the University of California, San Francisco.
American Academy of Pediatrics (AAP)
The AAP fosters dialogue among stakeholders and advocates for legislative changes to improve pediatric medical device development. Its Section on Advances in Therapeutics and Technology (SOATT) offers educational resources and engages members in activities related to medical devices and innovation.
Advanced Medical Technology Association (AdvaMed)
AdvaMed advocates for regulatory reforms and supports the development of pediatric-specific review teams. It also proposes solutions to regulatory agencies, such as designating pediatric devices as breakthrough products and accepting valid scientific evidence other than well-controlled trials.
Real-World Evidence and NEST
The National Evaluation System for Health Technology (NEST) leverages real-world data to support regulatory decision-making. This initiative aims to reduce the need for expensive trials by utilizing data from clinical registries, electronic health records, and medical billing claims.
System of Hospitals for Innovation in Pediatrics - Medical Devices (SHIP-MD)
SHIP-MD aims to enhance the pediatric medical device ecosystem by facilitating evidence generation, regulatory approval, and stakeholder interaction. This pre-consortium, funded by the FDA, includes multiple stakeholders such as the Critical Path Institute and AdvaMed.
Conclusion
Pediatric medical devices are crucial for the health and well-being of children, but their development and regulation face significant challenges. The unique clinical, technical, regulatory, and financial considerations associated with pediatric devices necessitate specialized solutions and collaborative efforts. While several initiatives have made progress in promoting pediatric medical device innovation, closing the gap between pediatric and adult devices will require broad cooperation across stakeholders. With the appropriate prioritization and funding, the future of pediatric medical device innovation holds promise for improving the lives of children worldwide.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Espinoza, Juan, et al. "Pediatric medical device development and regulation: current state, barriers, and opportunities." Pediatrics 149.5 (2022): e2021053390.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 The FDA classifies devices based on a three-tier, risk-based system. (Espinoza J., et al., 2022)
Fig.1 The FDA classifies devices based on a three-tier, risk-based system. (Espinoza J., et al., 2022)