Circular Design Strategies for Medical Devices
If you are interested in products and services related to the research phase in this field, please contact for further inquiries.
The healthcare sector, a cornerstone of modern society, is undergoing a transformative shift towards sustainability. The traditional linear model of production and consumption, characterized by the "take, make, dispose" approach, is increasingly being recognized as unsustainable. In response, the concept of a circular economy has gained traction, offering a more sustainable alternative. A circular economy aims to minimize waste and maximize resource efficiency by keeping products and materials in use for as long as possible. This model is particularly relevant to the medical device industry, where the need for innovation and sustainability is paramount.
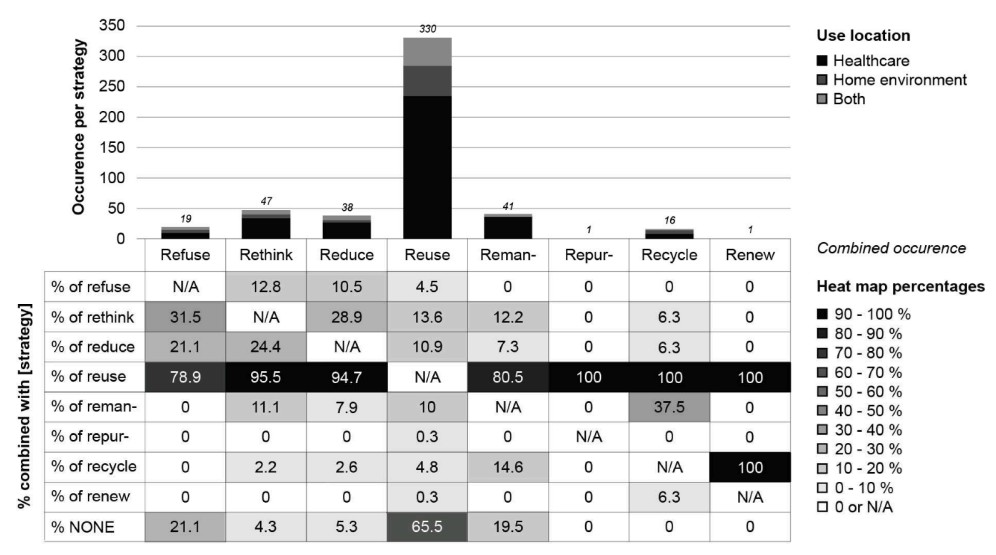 Fig.1 Occurrence per strategy and use location, and combined occurrence of multiple strategies. (Hoveling T., et al., 2024)
Fig.1 Occurrence per strategy and use location, and combined occurrence of multiple strategies. (Hoveling T., et al., 2024)
The Current Landscape of Medical Device Disposability
The medical device industry has long relied on single-use devices to ensure patient safety and prevent cross-contamination. However, this practice has led to a significant environmental burden. The healthcare sector's global climate footprint is greater than that of all aviation and shipping combined. The rapid advancement of digitization and electronics in healthcare has further exacerbated this issue, with electronic waste being one of the fastest-growing types of waste. The disposal of these devices not only contributes to environmental degradation but also represents a loss of valuable materials.
The Circular Economy Approach
- Circular Strategies in Medical Devices
The transition to a circular economy in the medical device industry involves implementing strategies such as reuse, remanufacturing, and recycling. These strategies aim to reduce waste and promote sustainability without compromising patient safety or device efficacy. A comprehensive study identified 346 active medical devices that employ at least one circular strategy, with reuse being the most common. However, other strategies such as reduce, rethink, and recycle are also essential for achieving a truly circular economy.
- The Hierarchy of Circular Strategies
Circular strategies can be ranked based on their environmental impact and feasibility. The hierarchy of circular strategies, from most to least favorable, includes refuse, rethink, reduce, reuse, remanufacture, repurpose, recycle, and recover energy. For instance, refusing to use a less sustainable device in favor of a more environmentally friendly alternative is considered the most favorable strategy. Conversely, recovering energy through incineration is the least favorable due to its environmental impact.
Barriers to Circular Transition
Safety and Infection Risks
One of the most significant barriers to the circular transition in medical devices is the concern over safety and infection risks. Ensuring that reused devices meet the same high standards of hygiene and safety as new ones is a complex challenge. Improper decontamination processes can lead to cross-contamination and pose a risk to patient safety. Addressing these concerns requires advanced decontamination methods and rigorous quality control.
Regulatory Constraints
Regulatory frameworks often prioritize patient safety over environmental sustainability, creating hurdles for circular practices. Regulations may not always align with the principles of a circular economy, making it difficult for manufacturers to implement circular strategies. Navigating these regulatory landscapes requires careful planning and collaboration between manufacturers, regulators, and healthcare providers.
Financial Limitations
The transition to a circular economy involves upfront costs, including investments in new technologies and processes. For many stakeholders, the financial incentives may not be immediately apparent, leading to resistance. However, long-term cost savings and environmental benefits can outweigh these initial challenges. For example, reusable devices can reduce the need for frequent replacements, leading to cost savings over time.
Technological Barriers
Not all medical devices are designed with circularity in mind. The complexity of modern devices, combined with the need for high performance and reliability, can make it difficult to implement circular strategies. This requires a redesign of devices and processes, which can be both technically and financially challenging. For instance, devices that are difficult to disassemble or contain hazardous materials pose significant challenges for recycling and remanufacturing.
Social and Systemic Barriers
Changing ingrained practices and attitudes is no small feat. Stakeholders, including healthcare providers, manufacturers, and patients, may be resistant to change due to familiarity with current practices or concerns about safety and efficacy. Overcoming these barriers requires education, collaboration, and a systemic approach. For example, training healthcare providers on proper decontamination and reuse procedures can help mitigate safety concerns.
Opportunities for a Circular Future
Policy and Regulation
Policies that encourage or mandate circular practices can create a supportive environment for change. This includes regulations that promote reuse, recycling, and remanufacturing, as well as incentives for manufacturers to design devices with circularity in mind. For example, the European Union has implemented regulations that require manufacturers to consider the environmental impact of their products throughout their lifecycle.
Technological Innovations
Advancements in technology can facilitate circular practices. For example, improved decontamination methods, advanced materials, and innovative design can make devices more suitable for reuse and recycling. Additionally, digital technologies such as device passports can enhance traceability and accountability in the circular lifecycle.
Financial Incentives
Creating financial incentives for circular practices can drive adoption. This includes business models that reward circularity, such as pay-per-use models or incentives for recycling. For example, companies that implement circular practices may receive tax breaks or subsidies, making these practices more financially viable.
Education and Collaboration
Education and training can help stakeholders understand the benefits of circular practices. Collaboration between manufacturers, healthcare providers, and policymakers can create a unified approach to implementing circular strategies. For example, joint training programs and workshops can help disseminate best practices and foster a culture of sustainability.
Design-Specific Recommendations
- Prioritize Quality and Safety
When implementing circular strategies, it is crucial to maintain the quality, function, and usability of medical devices. This ensures that circular practices do not compromise patient safety. For example, devices designed for reuse should undergo rigorous testing to ensure they meet the same standards as new devices.
- Combine Circular Strategies
Where possible, combine multiple circular strategies to maximize environmental benefits. For example, a device designed for reuse could also incorporate materials that are easy to recycle. This approach not only reduces waste but also enhances resource efficiency.
- Mitigate Safety Risks
Ensure that all circular strategies are implemented in a way that minimizes safety risks. This includes rigorous testing and validation of decontamination processes and materials. For example, devices that require frequent decontamination should be designed with materials that can withstand repeated cleaning and sterilization.
- Early Consideration of End-of-Life
Design devices with end-of-life in mind from the outset. This can include designing for disassembly, using recyclable materials, and planning for remanufacturing. For example, modular designs can facilitate easy disassembly and repair, making devices more suitable for circular strategies.
- Local Manufacturing and Distribution
Consider local manufacturing and distribution to minimize transportation emissions. This not only reduces the environmental impact but also supports local economies. For example, local recycling facilities can process medical waste more efficiently, reducing the need for long-distance transportation.
Conclusion: A Sustainable Future for Healthcare
The transition to a circular economy for medical devices presents both challenges and opportunities. By addressing the barriers and seizing the opportunities, we can create a more sustainable healthcare system. This requires collaboration, innovation, and a commitment to change. The future of healthcare is circular, and the benefits for both patients and the planet are immense.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Hoveling, Tamara, et al. "Circular economy for medical devices: barriers, opportunities and best practices from a design perspective." Resources, Conservation and Recycling 208 (2024): 107719.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Occurrence per strategy and use location, and combined occurrence of multiple strategies. (Hoveling T., et al., 2024)
Fig.1 Occurrence per strategy and use location, and combined occurrence of multiple strategies. (Hoveling T., et al., 2024)



