If you are interested in products and services related to the research phase in this field, please contact for further inquiries.
In the realm of medical device innovation, the development of BioAdheSil marks a significant leap forward in addressing the persistent challenge of securely anchoring silicone devices to biological tissues. Traditional adhesives often fall short when it comes to wet tissue environments, leading to device migration and potential complications. BioAdheSil, a novel silicone-based bioadhesive, has been meticulously engineered to overcome these limitations by providing robust adhesion in moist conditions, ensuring long-term stability and biocompatibility.
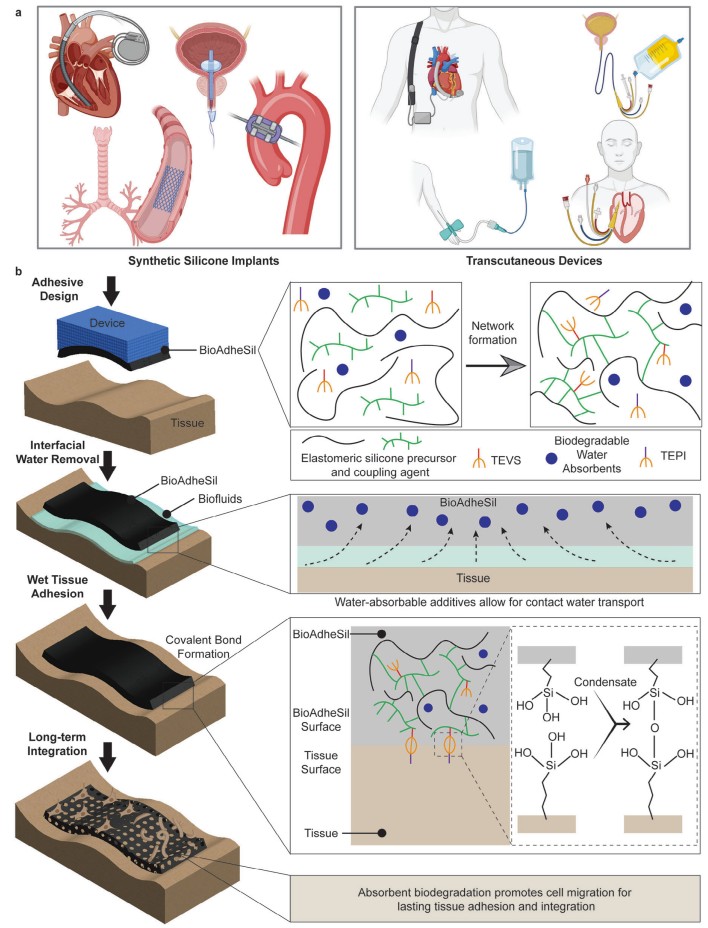 Fig.1 Overview and adhesive mechanism. a) Schematic illustrations demonstrate examples of diverse silicone medical devices that can be adhered to wet biological tissues using BioAdheSil, a silicone-based bioadhesive. b) Crosslinking mechanism and adhesive chemistry of BioAdheSil for bonding silicone devices to biological tissues. TEVS, triethoxy vinyl silane; TEPI, triethoxy(3-isocyanatopropyl)silane. (Singh M., et al., 2024)
Fig.1 Overview and adhesive mechanism. a) Schematic illustrations demonstrate examples of diverse silicone medical devices that can be adhered to wet biological tissues using BioAdheSil, a silicone-based bioadhesive. b) Crosslinking mechanism and adhesive chemistry of BioAdheSil for bonding silicone devices to biological tissues. TEVS, triethoxy vinyl silane; TEPI, triethoxy(3-isocyanatopropyl)silane. (Singh M., et al., 2024)
The Science Behind BioAdheSil
BioAdheSil's formulation is a sophisticated blend of soft silicone oligomers, siloxane coupling agents, and absorbents. This combination leverages the inherent biocompatibility of silicone while enhancing its ability to bond with hydrophilic tissues. The adhesive's design focuses on two critical aspects: wet tissue adhesion and tissue-infiltration-based long-term integration. The incorporation of biodegradable absorbents eliminates surface water and controls porosity, while silane crosslinkers provide interfacial strength. Over time, BioAdheSil transitions from a nonpermeable to a permeable state through enzyme degradation, creating a porous structure that facilitates cell migration and tissue integration.
Adhesion Mechanism and Performance
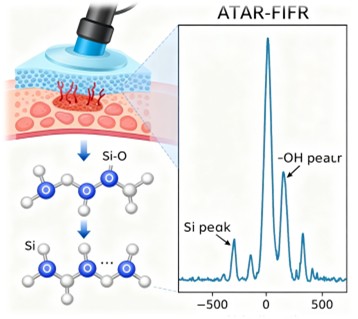
The adhesion mechanism of BioAdheSil is rooted in the formation of siloxane bonds at the interface between the silicone device and the biological tissue. This process is confirmed through attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR), which demonstrates the reduction in ─OH groups and the increase in Si─O bonds during curing. This chemical bonding ensures a strong and durable connection, even in wet conditions.
In terms of performance, BioAdheSil outperforms commercial adhesives, achieving an interfacial adhesion energy range of 800–1150 J/m² and a shear adhesion strength of 70–100 kPa between silicone and hydrated porcine skin. The adhesive's mechanical properties are comparable to those of soft tissues, with a tensile strength range of 600–1000 kPa and an elongation capability of up to 900%. This versatility makes BioAdheSil suitable for a wide range of medical applications.
Versatility in Medical Applications
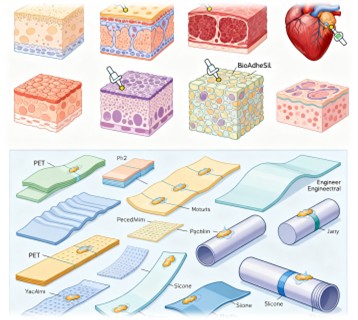
BioAdheSil's broad applicability is further demonstrated through its ability to adhere to a wide range of biological and synthetic materials. The adhesive has been tested on various tissues, including epidermis, subcutaneous tissue, intima, adventitia, endocardium, myocardium, and pericardium, as well as engineering substrates such as polyethylene terephthalate (PET), polyimide, thermoplastic polyurethane (TPU), silicone, cellulose, and Dacron polyester. The shear adhesion strength between silicone and wet tissue surfaces ranges from 37 kPa for the inner layer of the aorta to 114 kPa for epidermal skin.
This versatility makes BioAdheSil an ideal choice for applications such as tracheal stents, left ventricular assist devices (LVADs), and other long-term implants. The adhesive's ability to bond securely to both hydrophobic and hydrophilic surfaces ensures that medical devices remain in place, reducing the risk of migration and associated complications.
Biocompatibility and Safety
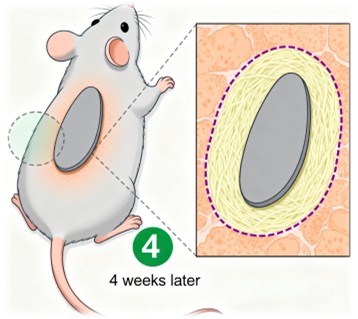
The biocompatibility of BioAdheSil is a critical aspect of its design. In vivo subcutaneous implantation in rats demonstrated that neither the starch-based nor the nonstarch-based BioAdheSil elicited adverse reactions after a 4-week implantation period. Histological analysis revealed the formation of a fibrous capsule around the implants, with no evidence of granulation tissue formation, necrosis, or allergic reactions.
Immunohistochemical (IHC) analysis using anti-CD68 antibodies showed no significant inflammatory response, indicating that BioAdheSil is well-tolerated by the body. This biocompatibility is crucial for ensuring that the adhesive does not cause adverse effects when used in medical applications, making it a safe and reliable choice for clinical use.
Preventing Device Migration
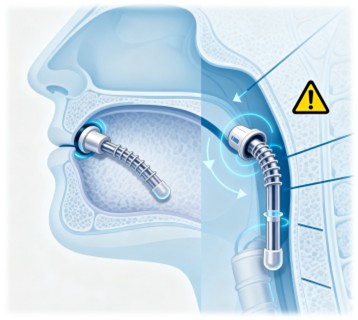
One of the key challenges in medical device implantation is preventing migration. Airway stents, for example, are prone to unintended dislocation, which can lead to serious complications. BioAdheSil offers a solution by providing strong adhesion between the stent and the tracheal tissue.
The effectiveness of BioAdheSil in securing three types of commercially available silicone stents was evaluated. The results showed that BioAdheSil significantly increased the antimigration force, making it a promising solution for preventing stent migration. Additionally, BioAdheSil can fill interfacial gaps, ensuring a secure fit even in cases where the stent size does not perfectly match the airway anatomy.
Infection Prevention in Transcutaneous Devices
Transcutaneous devices, such as LVAD drivelines, are prone to infections at the exit site. BioAdheSil can be used to create a biofluid-resistant adhesive barrier around the driveline, reducing the risk of microbial colonization and infection.
The adhesion strength of BioAdheSil was tested by securing the LVAD driveline to porcine skin. The results showed that BioAdheSil provided a strong bond, with a pull-off strength of 21±2 N for the silicone portion and 32±4 N for the velour region. This strong adhesion, combined with BioAdheSil's hydrophobic nature, makes it an effective barrier against biofilm migration.
Conclusion
BioAdheSil represents a significant advancement in the field of medical adhesives. Its unique formulation provides robust adhesion between silicone devices and wet biological tissues, addressing a critical challenge in medical device design. BioAdheSil's superior performance, broad applicability, and biocompatibility make it a versatile and reliable solution for a wide range of medical applications.
From securing airway stents to preventing infections at the exit site of transcutaneous devices, BioAdheSil has the potential to improve patient outcomes and enhance the performance of medical devices. As we continue to explore new applications and optimize its formulation, BioAdheSil stands as a testament to the power of innovation in medical technology.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Singh, Manisha, et al. "A tunable soft silicone bioadhesive for secure anchoring of diverse medical devices to wet biological tissue." Advanced Materials 36.3 (2024): 2307288.
This article is for research use only. Do not use in any diagnostic or therapeutic application.


