If you are interested in products and services related to the research phase in this field, please contact for further inquiries.
Coagulation testing is a critical component in the diagnosis and management of various hematological disorders. These tests provide essential insights into the clotting mechanisms of blood, aiding in the identification and treatment of conditions such as thrombosis, hemophilia, and other bleeding disorders. The accuracy and reliability of coagulation tests are significantly influenced by preanalytical variables (PAVs) and the presence of direct oral anticoagulants (DOACs). This article delves into the complexities of coagulation testing, focusing on the impact of PAVs and the challenges posed by DOACs.
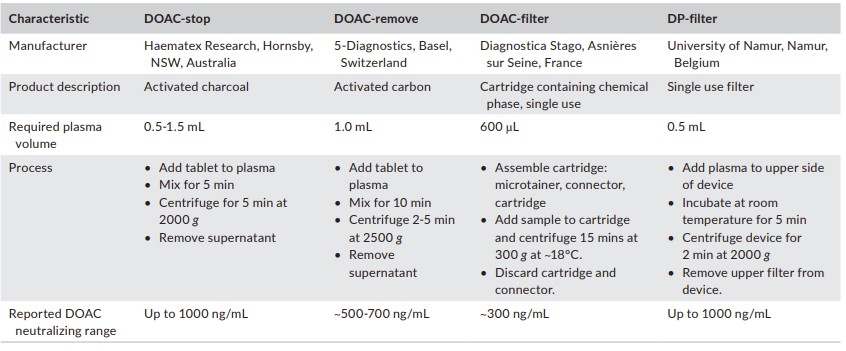 Fig.1 Comparison of commercially available direct oral anticoagulants (DOAC) neutralizing products. (Gosselin R. C., et al., 2021)
Fig.1 Comparison of commercially available direct oral anticoagulants (DOAC) neutralizing products. (Gosselin R. C., et al., 2021)
The Preanalytical Phase: Setting the Stage for Accurate Results
The preanalytical phase encompasses all steps from the moment a test is ordered until the sample reaches the laboratory for analysis. This phase is crucial as it can introduce significant variability into the test results. PAVs include patient selection, timing of blood collection, phlebotomy practices, sample transportation, processing, and storage. Errors during this phase are common and can lead to inaccurate coagulation test results, potentially affecting patient care.
Patient Factors and Timing
Patient-related factors such as age, gender, disease state, and therapeutic interventions can significantly influence coagulation test outcomes. For instance, neonatal protein C levels that are normal for a newborn might indicate severe deficiency in an adult. Similarly, females and males have different normal ranges for protein S levels. Conditions like pregnancy, hormonal cycles, liver function, inflammation, trauma, surgery, and even diet and exercise can impact hemostasis testing. Over-the-counter medications, herbal supplements, and certain antibiotics can also interfere with platelet function
Blood Collection Techniques
The method of blood collection is another critical PAV. Proper venipuncture techniques, the use of the correct needle size, and the order of tube collection are all essential. Most coagulation tests require blood anticoagulated with 3.2% sodium citrate. However, other anticoagulants like lithium heparin or acid citrate dextrose (ACD) may be used for specific tests. The source of blood collection tubes should be consistent, as differences between manufacturers can affect results. Blood collected via indwelling catheters requires clear instructions to avoid contamination or dilution.
Sample Transportation and Stability
Once collected, blood samples must be transported to the laboratory promptly and under the right conditions. Sample stability can range from 1 to 24 hours, depending on the test. Coagulation samples should generally be transported at room temperature. Extreme temperatures can affect sample integrity, so insulated transport mechanisms may be necessary. Pneumatic tube systems, while convenient, can impact platelet function and thrombin generation tests.
Laboratory Processing and Storage
Upon arrival at the laboratory, coagulation samples should be processed as soon as possible. Proper labeling and identification are crucial to avoid mix-ups. For plasma samples, centrifugation is required to achieve platelet-poor plasma (PPP). The centrifuge speed and temperature can affect the quality of the plasma. Samples that cannot be tested within the stability threshold should be stored appropriately. PPP can be stored in non-frost-free freezers at -20°C for up to two weeks or at -70°C for longer periods.
Sample Conditions and Handling
The physical traits of the processed sample, such as viscosity, hemolysis, icterus, and lipemia (HIL), can impact test results. Clotted plasma or hemolyzed samples often need to be recollected. Frozen samples must be thawed carefully to avoid increased viscosity. Modern coagulation analyzers can flag samples with HIL, but the impact of these conditions varies depending on the test method.
The Impact of Direct Oral Anticoagulants (DOACs)
The introduction of DOACs has revolutionized anticoagulant therapy but has also complicated coagulation testing. Unlike warfarin, DOACs do not require routine monitoring, but their presence can interfere with standard coagulation tests. This interference can lead to false-negative or false-positive results, potentially causing misdiagnosis or inappropriate treatment.
- DOACs and Diagnostic Testing
DOACs can interfere with tests used for diagnosing conditions like lupus anticoagulant (LA) or thrombophilia. For instance, rivaroxaban has been shown to be less effective than warfarin in treating antiphospholipid antibody syndrome (APS). Current recommendations suggest avoiding LA testing in patients on DOACs, but the use of DOAC neutralizing agents is being explored.
- Neutralizing Agents for DOACs
Activated charcoal has been shown to neutralize most DOACs effectively. Products like DOAC-Stop and DOAC-Remove use activated charcoal to remove DOACs from plasma samples. However, the effectiveness of these agents can vary based on the drug's molecular weight and charge. Post-treatment measurement of DOAC concentrations is recommended to ensure complete neutralization.
- Monitoring Anticoagulation in the Presence of DOACs
DOACs like dabigatran do not affect anti-Xa measurements, but FXa inhibitors like rivaroxaban and apixaban do. Institutions using heparin-calibrated anti-Xa measurements to screen for FXa inhibitors must be aware of the lack of standardization between different reagent platforms. In cases of concomitant therapy with heparins and DOACs, alternative strategies for monitoring anticoagulation are necessary.
Conclusion: Ensuring Accurate Coagulation Testing
Clinical laboratories play a pivotal role in ensuring the accuracy of coagulation tests. They must have detailed knowledge of PAVs and documented policies to address these variables. For patients on DOACs, laboratories must be aware of the potential interference and consider using neutralizing agents or alternative tests. Each institution should have a documented plan for handling samples affected by PAVs to prevent mismanagement or misdiagnosis. By following published guidelines and accreditation requirements, laboratories can optimize the accuracy of coagulation testing and contribute to better patient care.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Gosselin, Robert C. "Review of coagulation preanalytical variables with update on the effect of direct oral anticoagulants." International Journal of Laboratory Hematology 43 (2021): 109-116.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Comparison of commercially available direct oral anticoagulants (DOAC) neutralizing products. (Gosselin R. C., et al., 2021)
Fig.1 Comparison of commercially available direct oral anticoagulants (DOAC) neutralizing products. (Gosselin R. C., et al., 2021)