The diagnosis of autoimmune hemolytic anemia (AIHA) is challenging, requiring a meticulous, comprehensive approach rather than relying solely on a single test. This resource page provides an in-depth discussion of essential laboratory strategies for accurately identifying and classifying AIHA. The following sections provide a detailed roadmap of this diagnostic pathway, from initial suspicion to definitive diagnosis, and outline the important role and interpretation of each key test in the modern laboratory workflow.
Introduction to Autoimmune Hemolytic Anemia (AIHA)
Autoimmune hemolytic anemia (AIHA) is an acquired disorder in which autoantibodies trigger premature red blood cell destruction through complement activation and/or Fc-mediated phagocytosis. AIHA is characterized by the sudden or insidious onset of anemia, jaundice, and elevated reticulocyte counts and is categorized as warm, cold, or mixed based on the thermal amplitude and immunoglobulin profile of the causative antibodies. Distinguishing primary AIHA from secondary forms (such as those caused by concurrent lymphoproliferative disorders, systemic autoimmune diseases, infections, or drugs) is crucial for prognosis and treatment.
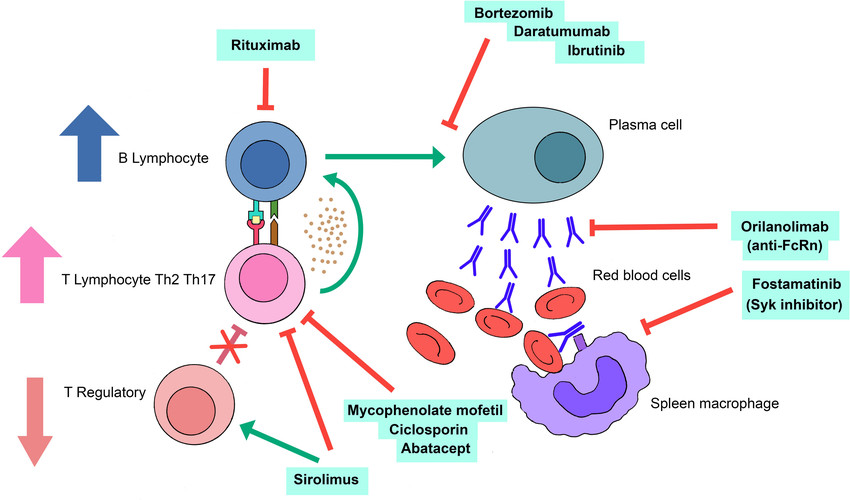 Fig.1 Pathogenesis of autoimmune hemolytic anemia (AIHA) post hematopoietic stem cell transplantation and potential therapeutic targets. (Gabelli M, et al., 2022)
Fig.1 Pathogenesis of autoimmune hemolytic anemia (AIHA) post hematopoietic stem cell transplantation and potential therapeutic targets. (Gabelli M, et al., 2022)
Clinical Suspicion of Autoimmune Hemolytic Anemia (AIHA)
Recognizing autoimmune hemolytic anemia (AIHA) begins with a high index of suspicion in any patient who presents with acute or progressive hemolytic anemia of unclear etiology.
Typical Symptoms
Typical clues include rapid-onset fatigue, dyspnea, or syncope accompanied by physical findings such as jaundice, scleral icterus, dark urine, or, in cold-type disease, acrocyanosis precipitated by cold exposure. A thorough medication and infection history is essential, because drugs (e.g., penicillins, cephalosporins, fludarabine) and pathogens (Mycoplasma pneumoniae, EBV, SARS-CoV-2) are well-established secondary triggers.
Red-Flag Symptoms
Laboratory "red flags" consist of normocytic or macrocytic anemia with an elevated reticulocyte count, unconjugated hyperbilirubinemia, increased lactate dehydrogenase, low haptoglobin, and evidence of spherocytes or red-cell agglutinates on the peripheral smear. In neonates or pregnant patients, AIHA can masquerade as early-onset jaundice or hydrops fetalis, mandating urgent evaluation. Clinicians should concurrently screen for lymphadenopathy, splenomegaly, arthralgias, or cytopenias.
Laboratory Testing for Autoimmune Hemolytic Anemia (AIHA)
The laboratory diagnosis of autoimmune hemolytic anemia (AIHA) is a multi-stage investigative process that progresses from confirming the presence of hemolysis to identifying its immune-mediated cause. It requires a structured approach, beginning with first-line screening tests to establish evidence of hemolysis and anemia, followed by second-line specialized serological tests to confirm the autoimmune etiology and classify the AIHA subtype. This stepwise strategy is crucial for accurate diagnosis, as no single test is sufficient on its own.
First-Line Testing: Establishing Hemolysis
Complete Blood Count (CBC) and Peripheral Blood Smear
The CBC and peripheral smear form the fundamental hematological assessment for AIHA. The CBC provides quantitative data revealing anemia and the bone marrow's compensatory response, which is typically evidenced by a markedly elevated reticulocyte count. Crucially, the peripheral smear offers qualitative, morphological evidence of immune-mediated damage; the presence of spherocytes is a classic hallmark of warm AIHA, while red blood cell agglutination is a pathognomonic sign of cold agglutinin disease.
Hemolytic Biomarkers
Biochemical markers provide functional evidence of active red blood cell destruction by measuring the byproducts of hemolysis. A significantly decreased or absent haptoglobin level is one of the most sensitive indicators, as this plasma protein is consumed when binding free hemoglobin released from lysed cells. Concurrent elevations in lactate dehydrogenase (LDH) and indirect bilirubin further confirm the presence and intensity of the hemolytic process, completing the laboratory picture of accelerated RBC turnover.
Second-Line Testing: Serological Confirmation and Subtyping
Direct Antiglobulin Test (DAT)
The DAT is the definitive test for confirming immune-mediated hemolysis. It detects the presence of bound antibodies (IgG) and/or complement components on the surface of the patient's red blood cells. A positive result is a serological hallmark of AIHA, and the specific pattern of reactivity provides the key initial data for AIHA classification.
Indirect Antiglobulin Test (IAT)
The IAT is used to detect unbound antibodies circulating in a patient's serum. Unlike the DAT, it identifies antibodies that may be bound to red blood cells but are not currently bound. In the context of AIHA, its primary role is to help determine the specificity and temperature range of free autoantibodies, which can provide additional information to support diagnosis and subtyping.
Cold Agglutinin Titer
This is a specialized test required for the diagnosis of cold agglutinin disease (CAD). The test measures the concentration of IgM autoantibodies in serum that agglutinate red blood cells at low temperatures. A clinically significant titer supports the diagnosis, but thermal amplitude is a more important indicator, as antibodies that react at higher temperatures are more likely to cause symptoms.
Future of Autoimmune Hemolytic Anemia (AIHA) Diagnostics
The future of autoimmune hemolytic anemia (AIHA) diagnostics is shifting towards integrated, multi-parameter strategies that move beyond the traditional direct antiglobulin test (DAT) to address seronegative cases and improve subclassification. This will be driven by the adoption of more sensitive solid-phase methodologies like ELISA and flow cytometry, complemented by the standardization of advanced genetic and molecular profiling. Ultimately, these innovations aim to facilitate earlier, more precise diagnosis and enable personalized treatment approaches tailored to the specific immunological drivers of the disease.
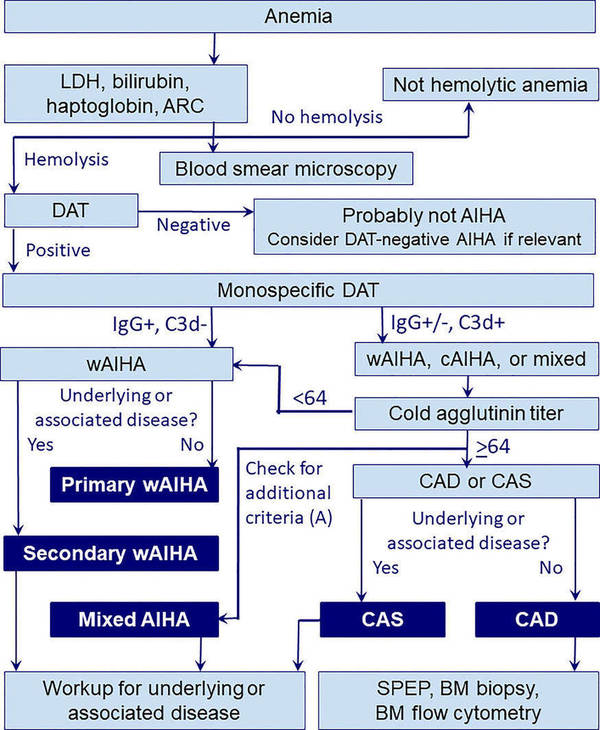 Fig.2 Diagnostic workup in autoimmune hemolytic anemia (AIHA). (Berentsen S, et al., 2023)
Fig.2 Diagnostic workup in autoimmune hemolytic anemia (AIHA). (Berentsen S, et al., 2023)
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) products for autoimmune hemolytic anemia (AIHA), spanning first-line hemolytic biomarkers, essential serological reagents for direct and indirect antiglobulin testing (DAT/IAT), and specialized solutions for cold agglutinin titer and advanced solid-phase detection. If you have related needs, please feel free to contact us for more information or product support.
References
- Gabelli M, Ademokun C, Cooper N, et al. Pathogenesis, risk factors and therapeutic options for autoimmune haemolytic anaemia in the post‐transplant setting[J]. British Journal of Haematology, 2022, 196(1): 45-62.
- Berentsen S, Fattizzo B, Barcellini W. The choice of new treatments in autoimmune hemolytic anemia: how to pick from the basket?[J]. Frontiers in immunology, 2023, 14: 1180509.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Pathogenesis of autoimmune hemolytic anemia (AIHA) post hematopoietic stem cell transplantation and potential therapeutic targets. (Gabelli M, et al., 2022)
Fig.1 Pathogenesis of autoimmune hemolytic anemia (AIHA) post hematopoietic stem cell transplantation and potential therapeutic targets. (Gabelli M, et al., 2022) 

 Fig.2 Diagnostic workup in autoimmune hemolytic anemia (AIHA). (Berentsen S, et al., 2023)
Fig.2 Diagnostic workup in autoimmune hemolytic anemia (AIHA). (Berentsen S, et al., 2023)