- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- From Newborn Screen to Genetic Confirmation: The Isovaleric Acidemia (IVA) Diagnostic Journey
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Isovaleric acidemia (IVA) is an inherited metabolic disorder disrupting the breakdown of the amino acid leucine. This resource provides a comprehensive guide to its diagnostic pathway, detailing the multi-stage process from initial suspicion to definitive genetic confirmation. We will explore the critical role of newborn screening, the specific biochemical biomarkers used for provisional diagnosis, and the advanced molecular techniques that deliver a conclusive result.
Isovaleric acidemia (IVA) is an inherited metabolic disorder characterized by the body's inability to properly break down the amino acid leucine. This defect is caused by a deficiency in the enzyme isovaleryl-CoA dehydrogenase, leading to the dangerous accumulation of toxic substances like isovaleric acid in the blood. IVA can present as a severe, life-threatening illness in newborns or with a more intermittent, chronic form in later infancy and childhood. Early diagnosis through newborn screening and confirmatory testing is critical, as it enables prompt initiation of dietary management and medical treatment to prevent metabolic crises and ensure better long-term health outcomes.
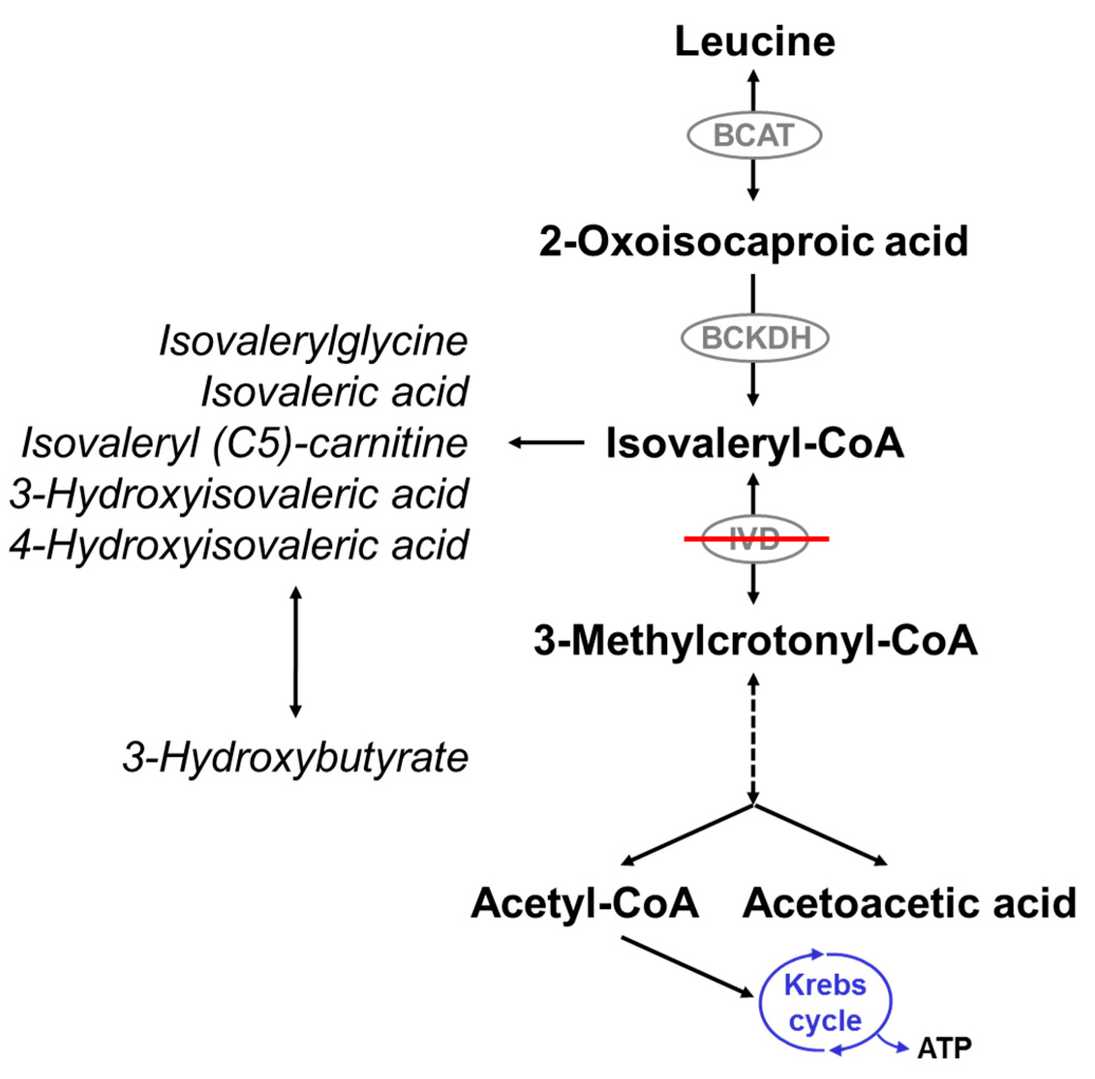 Fig.1 Leucine catabolism pathway. (Schlune A, et al., 2018)
Fig.1 Leucine catabolism pathway. (Schlune A, et al., 2018)
Newborn screening (NBS) serves as a critical public health initiative for the early detection of isovaleric acidemia (IVA) and other inherited metabolic disorders. The primary goal is to identify at-risk infants within the first few days of life, often before the onset of clinical symptoms, allowing for timely intervention to prevent severe and life-threatening complications.
The screening process for IVA is both highly specific and efficient:
It is crucial to understand that an abnormal NBS result is a screening alert, not a definitive diagnosis. It indicates the need for immediate and specialized follow-up testing.
Following an abnormal newborn screening (NBS) result suggestive of isovaleric acidemia (IVA), a rapid and systematic clinical and biochemical assessment is initiated to confirm the diagnosis. This critical phase moves from a screening alert to a definitive diagnostic workup.

A metabolic specialist will perform a clinical evaluation of infants with abnormalities found on newborn screening. This urgent assessment aims to identify any subtle signs of metabolic distress, even in seemingly well infants, including a thorough history of feeding and activity and a physical exam checking for a characteristic "sweaty feet" odor, lethargy, or poor tone. The primary goal is to determine the child's clinical status and rule out an impending metabolic crisis, guiding the speed and course of subsequent diagnostic steps.
Confirmatory biochemical testing provides the definitive laboratory evidence for IVA. This involves quantitative plasma acylcarnitine analysis to validate the persistent elevation of C5-isovalerylcarnitine and, most critically, urine organic acid analysis to detect the pathognomonic marker isovalerylglycine. The presence of this specific metabolite pattern is diagnostic for the enzymatic block in IVA, solidifying the diagnosis and enabling treatment to begin while genetic confirmation is pending.
Genetic confirmation represents the definitive diagnostic step for isovaleric acidemia (IVA), moving beyond biochemical markers to identify the underlying molecular cause of the disorder. This process involves targeted analysis of the IVD gene, which provides the instructions for producing the isovaleryl-CoA dehydrogenase enzyme. Through DNA sequencing techniques, typically using blood or saliva samples, clinical geneticists can identify biallelic pathogenic variants (disease-causing mutations inherited from both parents) within this gene. Identifying these mutations not only confirms the diagnosis with certainty but also enables crucial family studies. This includes carrier testing for relatives and providing options for prenatal diagnosis in future pregnancies, making genetic analysis an indispensable tool for both confirming IVA and guiding long-term family management.
The diagnosis of isovaleric acidemia (IVA) relies on a multi-tiered testing strategy. The following table outlines the common types of IVD products and assays used throughout the diagnostic journey.
| Diagnostic Tier | Target Analyte | Common IVD Product |
| Newborn Screening | C5-acylcarnitine (Isovalerylcarnitine) | Tandem mass spectrometry (MS/MS) kits, reagents for dried blood spot analysis |
| Biochemical Confirmation | Quantitative C5-acylcarnitine | MS/MS kits, calibrators for plasma/serum analysis |
| Isovalerylglycine, 3-Hydroxyisovalerate | Gas chromatography-mass spectrometry (GC-MS) kits, reagents for urine organic acid analysis | |
| Genetic Confirmation | IVD gene sequence or mutation | Next-generation sequencing (NGS) panels, sanger sequencing kits, targeted mutation analysis kits |
Alta DiagnoTech provides comprehensive, end-to-end IVD solutions for the diagnosis of metabolic disorders like isovaleric acidemia (IVA). Our integrated portfolio spans the entire diagnostic journey—from high-throughput newborn screening reagents and precise biochemical assays for detecting key biomarkers like isovalerylglycine, to definitive genetic confirmation using accredited NGS panels. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| AACAK-YJL-0011 | Glutamate Decarboxylase (GAD) Activity Assay Kit, 100T/48S | Add To Cart |
| AACAK-YJL-0008 | Leucine Arylamidase (LAP) Activity Assay Kit, 100T/96S | Add To Cart |
| AACAK-YJL-0016 | Proline (Pro) Content Assay Kit, 100T/96S, 200T/192S | Add To Cart |
| AACAK-YJL-0006 | Glutamate (Glu) Content Assay Kit (WST colorimetry), 50T/24S | Add To Cart |
| AACAK-YJL-0001 | Nithine-δ-aminotransferase (δ-OAT) Activity Assay Kit, 50T/48S | Add To Cart |
| AACAK-YJL-0009 | γ-Aminobutyric Acid (GABA) Content Assay Kit, 100T/96S | Add To Cart |
| AACAK-YJL-0014 | Cysteine (Cys) Content Assay Kit, 100T/96S | Add To Cart |
| AACAK-YJL-0022 | Glutamic Acid (Glu) Content Assay Kit, 100T/96S | Add To Cart |
| AACAK-YJL-0015 | Hydroxyproline (HYP) Content Assay Kit, 50T/48S | Add To Cart |
| AACAK-YJL-0024 | Glutamate (Glu) Content Assay Kit (WST colorimetry), 100T/48S | Add To Cart |
| AACAK-YJL-0020 | Micro Amino Acid (AA) Content Assay Kit, 100T/96S | Add To Cart |
| AACAK-YJL-0017 | Arginine (Arg) Content Assay Kit, 100T/96S | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |