- Home
- Resource
- Disease Diagnosis
- Autoimmune Diseases
- Closing the Diagnostic Gap: Advanced Serology in Systemic Lupus Erythematosus (SLE)
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Systemic lupus erythematosus (SLE) is a complex and heterogeneous autoimmune disease whose diagnosis remains challenging due to its diverse clinical manifestations and significant overlap with other conditions. This resource provides a comprehensive exploration of the modern diagnostic landscape for SLE, highlighting how advanced serological testing—integrated with clinical evaluation and emerging biomarkers—enables accurate detection, differential diagnosis, and monitoring of disease activity.
Systemic lupus erythematosus (SLE) is a chronic, systemic autoimmune disease characterized by the loss of immune tolerance and production of autoantibodies that can attack virtually any organ system, leading to widespread inflammation and tissue damage. Its presentation is highly heterogeneous, ranging from mild symptoms like fatigue and joint pain to severe, life-threatening complications involving the kidneys, heart, or central nervous system. Diagnosis remains challenging due to this variability and often relies on a combination of clinical criteria and serological biomarkers to confirm disease and guide treatment strategies.
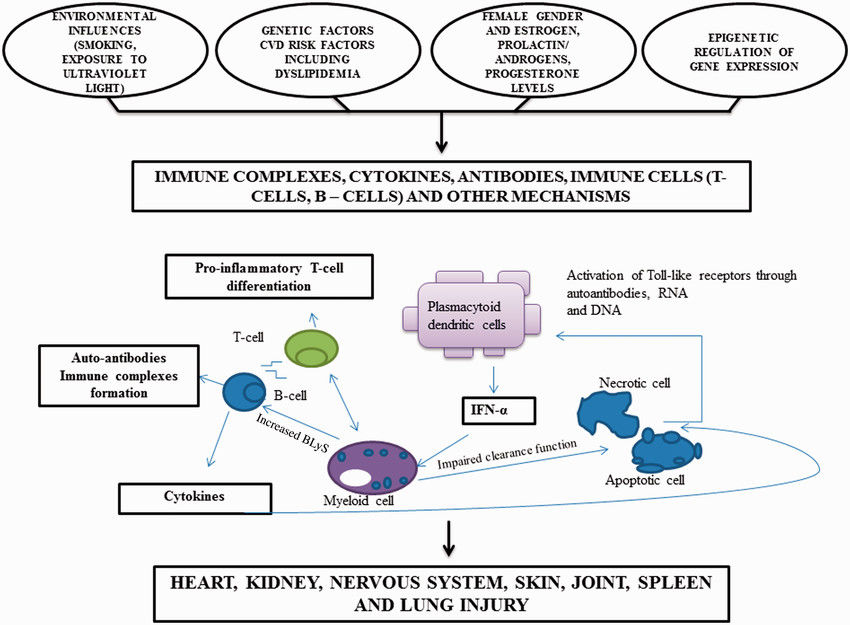 Fig.1 Diagram illustrating pathogenesis of systemic lupus erythematosus (SLE). (Matusik P S, et al., 2018)
Fig.1 Diagram illustrating pathogenesis of systemic lupus erythematosus (SLE). (Matusik P S, et al., 2018)
The antinuclear antibody (ANA) test is the primary and most sensitive serological screening tool for systemic autoimmune rheumatic diseases (SARDs), including systemic lupus erythematosus (SLE). It detects the presence of autoantibodies that target structures within the nucleus of a cell, a hallmark of autoimmunity. While highly sensitive for conditions like SLE (>95%), a positive ANA result is not diagnostic on its own, as it can also be found in other autoimmune diseases, infections, and even a small percentage of healthy individuals. Therefore, its primary clinical utility lies in raising suspicion for autoimmunity; a positive result must always be interpreted in the context of the patient's clinical symptoms and followed by more specific antibody testing (e.g., anti-dsDNA, anti-ENA) to either confirm a diagnosis or rule out disease.
While the antinuclear antibody (ANA) test serves as a sensitive screening tool for autoimmunity, specific autoantibody testing is critical for confirming the diagnosis of systemic lupus erythematosus (SLE), differentiating it from other conditions, and assessing clinical subsets and disease activity. These tests target particular nuclear and cytoplasmic antigens and provide the objectivity and specificity required to close the diagnostic gap in patients with suggestive clinical features. Key autoantibodies include:
Anti-double-stranded DNA (anti-dsDNA):
Highly specific for SLE and often correlated with disease activity, particularly lupus nephritis. Rising titers may predict flares, making this antibody a valuable tool for monitoring disease progression and treatment response.
Anti-Smith (anti-Sm):
Considered one of the most specific markers for SLE, though it is not associated with disease activity. Its presence strongly supports the diagnosis.
Anti-ribonucleoprotein (anti-RNP):
Found in SLE and other autoimmune diseases like mixed connective tissue disease (MCTD). High titers may suggest overlapping features.

Anti-Ro/SSA and Anti-La/SSB:
Commonly associated with Sjögren's syndrome but also prevalent in SLE. Linked to subphenotypes such as photosensitivity, sicca symptoms, neonatal lupus, and subacute cutaneous lupus.
Antiphospholipid Antibodies (aPL):
Including lupus anticoagulant (LA), anticardiolipin antibodies (aCL), and anti-β₂-glycoprotein I (anti-β₂GPI). These identify patients at risk for thrombotic events, obstetric complications, and antiphospholipid syndrome (APS).
The differential diagnosis for systemic lupus erythematosus (SLE) is broad and critical due to its heterogeneous clinical presentation that overlaps with numerous autoimmune, inflammatory, infectious, and hematologic disorders. Key conditions to consider include:
A methodical approach combining clinical history, physical exam, serologic testing (e.g., ANA, anti-dsDNA, anti-Sm, and disease-specific antibodies), and targeted imaging or biopsy is essential to accurately distinguish SLE from its mimics and avoid misdiagnosis.
The future of systemic lupus erythematosus (SLE) diagnostics lies in moving beyond traditional serology toward multimodal, precision-driven approaches, integrating novel biomarkers, such as interferon signatures, cell-bound complement activation products (CB-CAPs), and proteomic/metabolic profiles, with advanced technologies like artificial intelligence (AI) for pattern recognition in imaging and multi-omics data. These innovations aim to address current limitations in sensitivity and specificity, particularly in seronegative and early-stage disease, while enabling earlier detection, stratification of heterogeneous subtypes, and dynamic monitoring of disease activity and treatment response.
Leveraging extensive expertise and cutting-edge technology, Alta DiagnoTech offers a comprehensive IVD solution for systemic lupus erythematosus (SLE), encompassing ANA screening assays, specific autoantibody tests (anti-dsDNA, anti-Sm, etc.), complement profiling kits, and novel biomarker panels. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| EK-YJL-3918 | Human Anti-double Stranded DNA (dsDNA) Antibody (IgM) ELISA Kit | Add To Cart |
| EK-YJL-1667 | Human Anti-double Stranded DNA (dsDNA) Antibody (IgG) ELISA Kit | Add To Cart |
| EC-0020 | Murine MCP-1 ELISA Kit | Add To Cart |
| EC-0034 | Human IL-13 ELISA Kit | Add To Cart |
| EC-0019 | Murine IP-10 ELISA Kit | Add To Cart |
| EC-0025 | Rat IL-10 ELISA Kit | Add To Cart |
| EC-0044 | Human p53 ELISA Kit | Add To Cart |
| CA-00163 | Typhoid IgG/IgM rapid test kit | Add To Cart |
| CA-00161 | Filariasis IgG/IgM rapid assay kit | Add To Cart |
| EC-0018 | Murine IL-22 ELISA Kit | Add To Cart |
| EC-0026 | Rat IL-17A ELISA Kit | Add To Cart |
| EC-0048 | Human CD117 ELISA Kit | Add To Cart |
| EC-0065 | Human IL-10 HS ELISA Kit | Add To Cart |
| EC-009 | Murine IL-4 ELISA Kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |