- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- Precision Diagnostics for Galactosemia: Key Biomarkers and Analytical Techniques
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Galactosemia is a rare but critical inherited metabolic disorder where the body's inability to process galactose can lead to severe complications without early intervention. Precision in diagnosis is paramount, relying on a multi-faceted approach that integrates biochemical assays, genetic analysis, and ongoing metabolic monitoring. This article delves into the essential biomarkers, advanced analytical techniques, and step-by-step diagnostic pathway for galactosemia, highlighting how modern technology enables accurate detection and personalized management.
Galactosemia is a rare, inherited metabolic disorder characterized by the body's inability to effectively metabolize galactose, a sugar found primarily in dairy products. This condition arises from deficient activity of enzymes involved in the galactose metabolism pathway, most commonly galactose-1-phosphate uridylyltransferase (GALT). Accumulation of toxic metabolites like galactose-1-phosphate can lead to severe complications, including liver damage, renal dysfunction, cataracts, and intellectual disability, if not diagnosed and managed early.
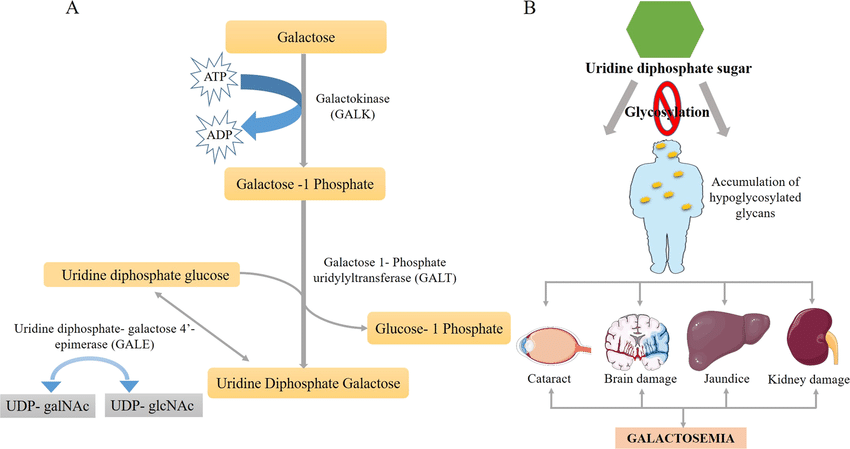 Fig.1 Intracellular cystine metabolism. (Bäumner S, Weber L T., 2018)
Fig.1 Intracellular cystine metabolism. (Bäumner S, Weber L T., 2018)
The precise diagnosis of galactosemia relies on the measurement of specific biochemical and genetic biomarkers. These biomarkers form a multi-tiered diagnostic strategy, moving from initial screening to definitive confirmation.

This is the primary functional biomarker. Measured in erythrocytes (red blood cells) or dried blood spots (DBS), a severely deficient or absent GALT enzyme activity is the hallmark of classic galactosemia. It confirms the metabolic block and is crucial for differentiating classic disease from variant forms (e.g., Duarte variant) which show partial activity.
This is the primary toxic metabolite that accumulates upstream of the metabolic block. Elevated erythrocyte Gal-1-P levels are a key diagnostic indicator and are also used to monitor dietary compliance and metabolic control throughout the patient's life, making it an essential tool for long-term management.

Molecular genetic testing for pathogenic variants in the GALT gene provides the definitive confirmatory diagnosis. Identifying biallelic pathogenic mutations (e.g., p.Gln188Arg, p.Lys285Asn) not only confirms the disease but also allows for carrier testing within families and can sometimes offer insights into potential disease severity.
The accurate diagnosis and monitoring of galactosemia depend on sophisticated analytical techniques designed to measure its key biomarkers. The choice of technique is dictated by the biomarker itself, the required sensitivity and specificity, and the context of testing (e.g., high-throughput newborn screening vs. confirmatory testing).

Techniques for Measuring GALT Enzyme Activity
GALT enzyme activity is primarily measured using enzymatic coupled assays, where the minimal activity in a patient sample triggers a reaction that produces a measurable signal. Fluorometric assays are highly sensitive and commonly used for high-throughput newborn screening with dried blood spots (DBS), while spectrophotometric (UV/Vis) assays provide robust quantitative confirmation of enzyme levels in erythrocyte samples, helping distinguish classic galactosemia from variant forms.
Techniques for Quantifying Galactose-1-Phosphate (Gal-1-P)
Galactose-1-phosphate (Gal-1-P) is quantified using highly specific analytical methods. Tandem mass spectrometry (MS/MS) is the gold standard, especially for newborn screening and monitoring, due to its exceptional sensitivity, specificity, and ability to analyze multiple metabolites simultaneously from dried blood spots. Alternatively, enzymatic assays offer a cost-effective and reliable solution for measuring Gal-1-P levels in erythrocytes during long-term treatment monitoring.
Techniques for Genetic Analysis (GALT Gene)
Genetic confirmation of galactosemia relies on DNA-based techniques to identify pathogenic variants in the GALT gene. Sanger sequencing is the gold standard for targeted analysis of the entire coding region, offering high accuracy for detecting point mutations and small insertions/deletions. For broader screening or complex cases, next-generation sequencing (NGS) panels enable efficient analysis of GALT alongside other metabolic genes, while MLPA is used to detect larger exon or gene deletions not visible through sequencing.
The diagnosis of galactosemia is not reliant on a single test but on a integrated, multi-tiered pathway that strategically combines biochemical and genetic biomarkers. This structured approach ensures rapid detection, accurate confirmation, and effective long-term management, guiding clinical intervention from the newborn period onward.
The future of galactosemia diagnostics lies in the integration of advanced technologies such as next-generation sequencing (NGS) and artificial intelligence (AI) to enable earlier, more precise, and personalized diagnoses. Emerging methods may include non-invasive prenatal testing and ultra-sensitive biosensors for real-time monitoring of metabolite levels. Additionally, the expansion of newborn screening panels and the adoption of multi-omics approaches (genomic, proteomic, metabolomic) will enhance our understanding of disease variants and facilitate tailored therapeutic strategies.
Alta DiagnoTech provides a comprehensive portfolio of IVD solutions for galactosemia, spanning from newborn screening and biochemical confirmation to genetic testing and long-term therapeutic monitoring. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |