- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- Biomarker Insights: The Acylcarnitine Profile as a Cornerstone for Diagnosing CPT II Deficiency
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Carnitine palmitoyltransferase II (CPT II) deficiency is a rare, inherited disorder of mitochondrial long-chain fatty acid oxidation. Prompt and accurate diagnosis is crucial for preventing life-threatening metabolic crises and guiding long-term management. This resource page provides an in-depth discussion of the diagnostic pathway for CPT II deficiency, focusing on acylcarnitine profiling and its integration into modern diagnostic workflows.
Carnitine palmitoyltransferase II (CPT II) deficiency is an autosomal recessive disorder of mitochondrial long-chain fatty acid oxidation (FAO). It disrupts the transport of long-chain fatty acids into the mitochondria, which are crucial for energy production, particularly during periods of fasting, illness, or exercise. The disorder manifests in three primary forms: a lethal neonatal multi-systemic type, a severe infantile hepatocardiomuscular type, and a more common myopathic adult-onset type characterized by recurrent episodes of exercise-induced rhabdomyolysis and muscle pain.
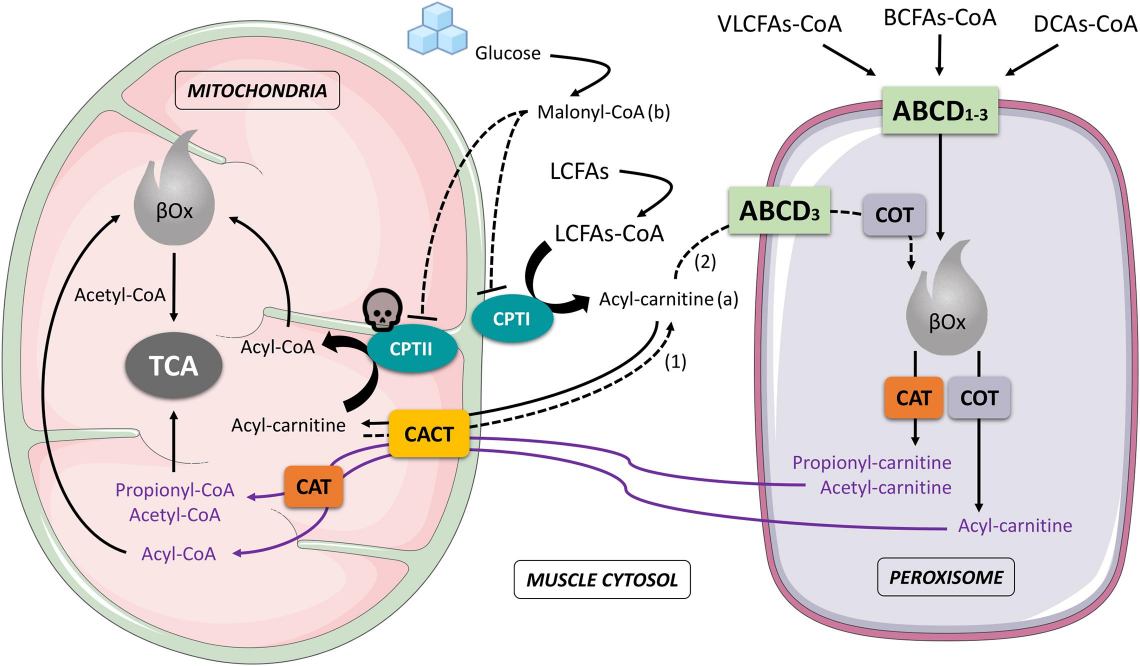 Fig.1 Schematic interaction that involves mitochondria and peroxisome during muscle cell fatty acids oxidation. (Negro M, et al., 2021)
Fig.1 Schematic interaction that involves mitochondria and peroxisome during muscle cell fatty acids oxidation. (Negro M, et al., 2021)
The acylcarnitine profile is a cornerstone biochemical test for diagnosing carnitine palmitoyltransferase II (CPT II) deficiency, providing a direct window into the underlying metabolic block. The key biomarkers are characteristically elevated levels of specific long-chain acylcarnitine species-namely C16, C18:1, and C18:2. This accumulation occurs due to the impaired mitochondrial import of long-chain fatty acids, causing them to conjugate with carnitine and leak into the bloodstream. Furthermore, the calculated ratio of free carnitine (C0) to the sum of these long-chain derivatives (C16 + C18) is significantly lowered, creating a highly sensitive and specific diagnostic signature that reliably differentiates CPT II Deficiency from other fatty acid oxidation disorders.
Integrating the acylcarnitine profile into the diagnostic workflow is fundamental for the efficient and accurate identification of carnitine palmitoyltransferase II (CPT II) deficiency. This powerful biochemical assay acts as a critical decision point, guiding clinicians from initial suspicion toward a definitive molecular diagnosis. Its integration ensures a streamlined path from screening to confirmation, enabling rapid intervention and management for affected individuals.

Newborn Screening (NBS)
In newborn screening (NBS), acylcarnitine profile analysis via tandem mass spectrometry (MS/MS) of dried blood spots serves as a critical first-tier tool for the early detection of CPT II Deficiency. A positive NBS result is not diagnostic but triggers urgent follow-up testing, enabling pre-symptomatic intervention, parental education, and the prevention of life-threatening metabolic crises.
Diagnostic Testing for Symptomatic Patients
While the acylcarnitine profile provides a highly specific biochemical diagnosis, confirmatory testing is essential to definitively establish a diagnosis of carnitine palmitoyltransferase II (CPT II) deficiency. This step moves from identifying the characteristic metabolic pattern to pinpointing the exact molecular cause, which is crucial for genetic counseling, prenatal diagnosis, and in some cases, clarifying prognosis.

Molecular genetic testing is the contemporary gold standard for confirming a diagnosis of CPT II Deficiency. This involves sequencing the CPT2 gene to identify pathogenic variants (mutations) on both alleles. Targeted analysis often first checks for common mutations, such as the p.Ser113Leu variant frequently associated with the adult-onset muscular form, followed by full gene sequencing if necessary. Identifying biallelic pathogenic variants provides definitive genetic confirmation of the disorder, allowing for carrier testing within the family and enabling precise genetic counseling.

The enzyme activity assay is a biochemical method that directly measures the activity of the CPT2 enzyme, typically in cultured fibroblasts or lymphocytes. A significantly reduced enzyme activity level confirms a defect in the CPT2 enzyme complex. While historically the definitive test, this assay has been largely superseded by more accessible and precise genetic testing. It is now primarily used in diagnostically complex cases or when genetic test results are inconclusive, providing functional evidence to support the molecular findings.
The future of carnitine palmitoyltransferase II (CPT II) deficiency diagnostics lies in the integration of advanced genomic sequencing and artificial intelligence. Next-generation sequencing is evolving into a first-line tool for comprehensive genetic confirmation, while AI-enhanced analysis of acylcarnitine profiles promises greater pattern recognition accuracy. These innovations, coupled with refined functional assays for variant interpretation, will accelerate precise diagnosis and enable more personalized treatment approaches.
Alta DiagnoTech provides comprehensive diagnostic solutions for carnitine palmitoyltransferase II (CPT II) deficiency, including specialized MS/MS-based acylcarnitine profiling kits and CPT2 genetic mutation detection reagents. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |