- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Navigating Gonorrhea Diagnostics: From NAATs to Resistance Testing
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Gonorrhea's growing antimicrobial resistance and high asymptomatic rates demand accurate, multi-method diagnostics. This resource details essential laboratory approaches, from gold-standard NAATs and culture-based AST to resistance biomarkers and emerging technologies. This expertise can help optimize testing, guide treatment, and monitor antimicrobial resistance to address this evolving pathogen.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is a globally prevalent sexually transmitted infection (STI) with rising antimicrobial resistance (AMR) concerns. Transmitted through sexual contact, it frequently presents asymptomatically (especially in women), but can lead to severe complications like pelvic inflammatory disease, infertility, and disseminated infections if untreated. Diagnosis relies on NAATs (nucleic acid amplification tests) for high sensitivity, supplemented by culture for antibiotic susceptibility testing to address multidrug-resistant strains.
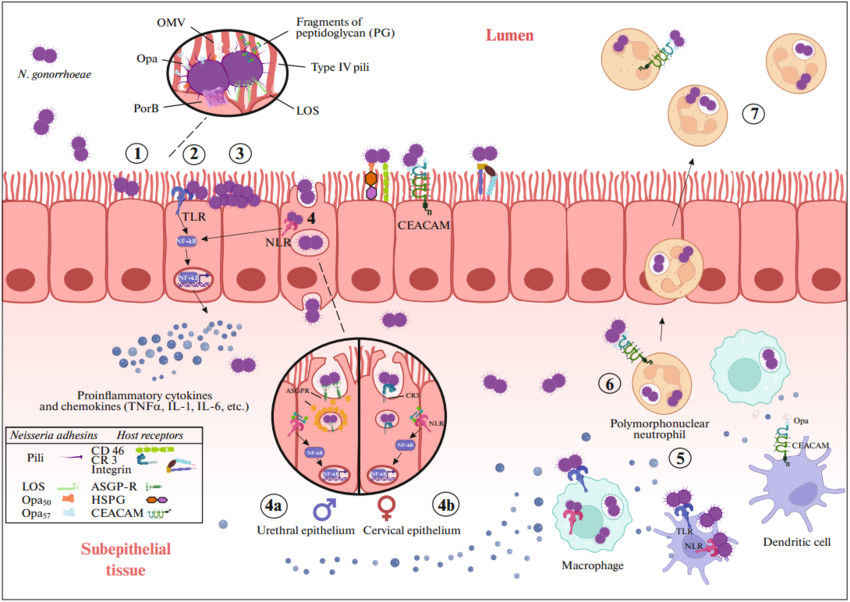 Fig.1 Pathogenesis of Neisseria gonorrhoeae infection. (Shaskolskiy B L, et al., 2024)
Fig.1 Pathogenesis of Neisseria gonorrhoeae infection. (Shaskolskiy B L, et al., 2024)
With over 82 million new cases annually (WHO 2020) and shrinking treatment options, accurate diagnostics are critical for both clinical management and public health surveillance to curb transmission and antimicrobial resistance (AMR) spread. A structured diagnostic workflow for gonorrhea is important for ensuring timely detection, guiding effective treatment, and supporting public health surveillance.
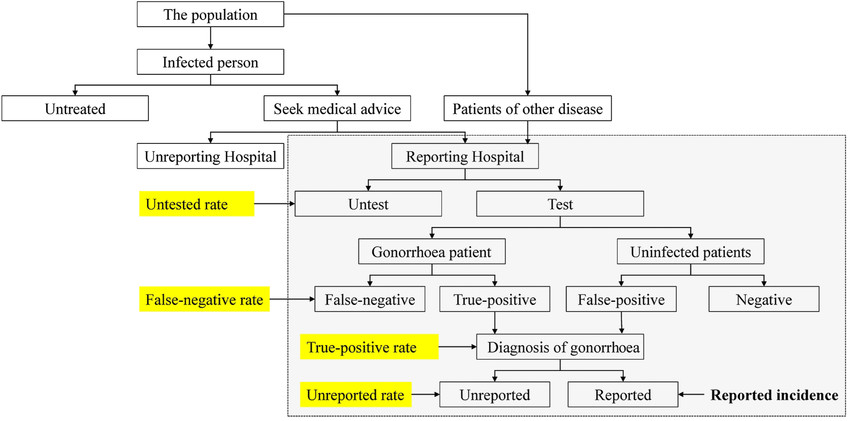 Fig.2 The clinical process of gonorrhea diagnosis. (Xiong M, et al., 2016)
Fig.2 The clinical process of gonorrhea diagnosis. (Xiong M, et al., 2016)
Accurate diagnosis of Neisseria gonorrhoeae requires a multi-method approach to address diverse clinical scenarios, from routine screening to antimicrobial resistance (AMR) surveillance. The three primary diagnostic pillars, NAATs, culture-based AST, and rapid tests, each play distinct roles in detection accuracy, treatment guidance, and point-of-care utility.
NAATs represent the gold standard for gonorrhea detection, offering >95% sensitivity by targeting conserved bacterial genes (porA, opa, or 16S rRNA). These tests excel in non-invasive sampling (urine, vaginal/urethral swabs) and multiplex formats, delivering results within hours. Their limitations include inability to assess AMR and potential cross-reactivity with commensal Neisseria species.

While slower (3–5 days), culture remains essential for AMR surveillance, isolating viable bacteria for phenotypic resistance profiling (e.g., against ceftriaxone or azithromycin). It is the only method that provides actionable AST data but suffers from stringent transport/storage requirements and lower sensitivity (~50–80%) compared to NAATs.

Lateral flow assays and other rapid tests enable point-of-care screening (15–30 minutes) but have limited sensitivity (~50–70%) and cannot differentiate active infections from past exposures. Emerging technologies aim to address these gaps for resource-limited settings.
The rise of multidrug-resistant Neisseria gonorrhoeae has made resistance detection as critical as pathogen identification. This section explores the molecular markers driving resistance and the assays revolutionizing surveillance.
Key Resistance Biomarkers
Gonorrhea's antimicrobial resistance (AMR) is driven by specific genetic mutations, including penA (ceftriaxone resistance), 23S rRNA (azithromycin resistance), and gyrA/parC (fluoroquinolone resistance). These biomarkers can enable molecular detection of resistant strains and guide targeted therapy.
Resistance Detection Technologies
Modern diagnostic platforms such as real-time PCR and whole-genome sequencing (WGS) enable rapid identification of resistance markers, delivering results significantly faster than traditional culture methods while enhancing both epidemiological surveillance and personalized treatment strategies.
The future of gonorrhea diagnostics is evolving toward integrated, rapid, and resistance-aware solutions, combining ultrasensitive molecular detection with real-time AMR profiling to address the growing antimicrobial resistance crisis. Next-generation platforms like fully automated POC NAAT systems and AI-powered genomic resistance prediction will enable same-visit diagnosis and tailored therapy, while cloud-connected diagnostic networks could transform regional AMR surveillance.
Alta DiagnoTech delivers comprehensive gonorrhea diagnostic products, from high-precision NAAT kits to innovative resistance detection assays, empowering clinicians and laboratories to combat this evolving STI threat with confidence. If you have related needs, please feel free to contact us for more information or product support.
References
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |