- Home
- Resource
- Disease Diagnosis
- Autoimmune Diseases
- Myasthenia Gravis Diagnostics Essential Biomarkers, Technologies, and Protocols
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Myasthenia gravis (MG) is an autoimmune disorder characterized by fatigable muscle weakness, whose diagnosis requires a meticulous integration of clinical evaluation, serological profiling, and electrophysiological confirmation. This resource details the essential biomarkers, including anti-AChR, anti-MuSK, and anti-LRP4 antibodies, and the advanced technologies (e.g., RIA, CBA, CLIA) used to detect them, enabling precise disease subtyping and personalized treatment strategies.
Myasthenia gravis (MG) is an autoimmune disorder characterized by antibodies targeting proteins at the neuromuscular junction, leading to impaired nerve-to-muscle communication and causing fatigable muscle weakness. Common symptoms include ptosis, diplopia, difficulty swallowing, and limb weakness, which worsen with activity and improve with rest. Diagnosis relies on a combination of clinical evaluation, serological detection of specific autoantibodies (e.g., against AChR, MuSK, or LRP4), and electrophysiological studies to confirm synaptic dysfunction.
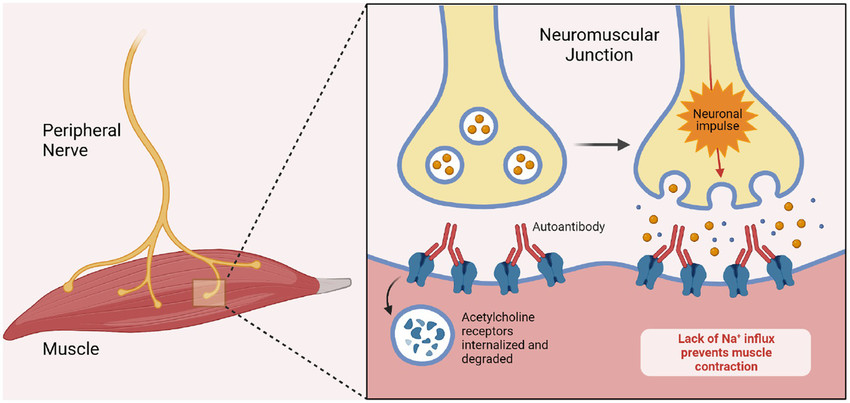 Fig.1 Pathogenesis of myasthenia gravis. (Gomez F, et al., 2023)
Fig.1 Pathogenesis of myasthenia gravis. (Gomez F, et al., 2023)
The serological diagnosis of myasthenia gravis (MG) relies on detecting specific autoantibodies that target key proteins at the neuromuscular junction, providing direct evidence of autoimmune pathology. These biomarkers are not only critical for confirming the diagnosis but also for defining clinical subtypes, predicting disease severity, and guiding personalized treatment strategies. The identification of these antibodies transforms MG from a clinically suspected syndrome into a precisely characterized autoimmune disorder, enabling targeted therapeutic interventions and improved patient outcomes.
Anti-acetylcholine receptor (AChR) antibodies are the most prevalent biomarker in MG, detected in approximately 85% of generalized cases. These antibodies directly target postsynaptic ACh receptors, leading to receptor blockade, complement-mediated destruction, and accelerated degradation, ultimately disrupting neuromuscular transmission. Testing for anti-AChR antibodies remains the first-line diagnostic approach due to its high disease specificity and clinical utility.
Anti-muscle-specific kinase (MuSK) antibodies define a distinct MG subtype, accounting for a subset of patients negative for AChR antibodies. These IgG4-dominated antibodies impair agrin-mediated AChR clustering, leading to simplified synaptic structures and severe neuromuscular dysfunction. Detection of anti-MuSK antibodies is essential for explaining seronegative cases and guiding tailored immunosuppressive therapies.
Anti-LRP4 antibodies are identified in a small proportion of patients who are seronegative for both AChR and MuSK antibodies, further reducing the diagnostic gap in MG. These antibodies target low-density lipoprotein receptor-related protein 4 (LRP4), a coreceptor for agrin that facilitates MuSK activation and AChR clustering. Testing for anti-LRP4 antibodies provides serological confirmation in double-negative cases and contributes to a more comprehensive molecular classification of autoimmune MG.
The accurate detection of myasthenia gravis (MG)-specific autoantibodies relies on a range of immunoassay technologies, each with distinct advantages in sensitivity, specificity, and clinical applicability. These methods form the foundation of serological testing, enabling the precise identification of anti-AChR, anti-MuSK, and anti-LRP4 antibodies to confirm diagnosis and guide subtype classification. The choice of assay depends on the target antibody, available laboratory infrastructure, and the need for quantitative accuracy, throughput, or avoidance of radioactive materials.
Radioimmunoprecipitation Assay (RIA)
RIA is historically considered the gold standard for detecting anti-AChR antibodies due to its high sensitivity and specificity. This method uses radioisotope-labeled human AChR antigens to immunoprecipitate patient antibodies, allowing precise quantification. Despite its diagnostic reliability, RIA requires specialized handling of radioactive materials and is increasingly replaced by non-radioactive alternatives in routine clinical practice.
Cell-Based Assays (CBA)
CBA is the preferred method for detecting anti-MuSK and anti-LRP4 antibodies, which often target conformational epitopes. By expressing full-length human antigens on live or fixed cell surfaces, CBA preserves native protein structures and provides high clinical specificity. This technology is particularly valuable for confirming seronegative MG cases and defining antibody subtypes with therapeutic implications.
Indirect Immunofluorescence (IIF)
IIF is a versatile technique that can screen for multiple neural and muscle antibodies using tissue substrates (e.g., primate cerebellum or muscle). While less specific for MG than antigen-specific assays, it offers a broad antibody overview and may aid in identifying rare neural targets. Its role in MG is largely supplemental to targeted serological tests.

Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA provides a robust, non-radioactive platform for anti-AChR antibody detection using purified antigen-coated plates and enzyme-linked detection. It offers excellent automation capability, standardized protocols, and high throughput, making it suitable for large-scale screening. However, sensitivity may be marginally lower than RIA for low-titer samples.
Chemiluminescence Assay (CLIA)
CLIA represents an advanced evolution of ELISA, utilizing chemiluminescent substrates for signal detection to achieve higher sensitivity and broader dynamic range. This technology is well-suited for modern automated platforms, enabling rapid, quantitative anti-AChR antibody testing with excellent reproducibility. CLIA is increasingly adopted as a non-radioactive alternative with performance comparable to RIA.
The diagnosis of myasthenia gravis (MG) follows a structured, stepwise protocol that integrates clinical assessment with sequential laboratory and electrophysiological testing to confirm autoimmune etiology and demonstrate neuromuscular junction dysfunction. This algorithm ensures efficient resource utilization while maximizing diagnostic sensitivity and specificity, particularly in seronegative or atypical cases. The following steps outline the standardized approach endorsed by clinical guidelines, emphasizing the critical role of antibody testing and neurophysiological studies in achieving a definitive diagnosis.
A thorough history and physical exam focusing on hallmark features like fatigable muscle weakness (e.g., ptosis, diplopia, dysphagia) is the essential first step to establish clinical suspicion.
Anti-AChR antibody testing (via high-sensitivity assays like RIA, ELISA, or CLIA) serves as the primary serological tool to confirm autoimmune MG in most generalized cases.
For patients with high clinical suspicion but negative AChR antibodies, testing for anti-MuSK antibodies using cell-based assays (CBA) is critical to identify this distinct MG subtype.
In double-negative (AChR-/MuSK-) cases, consider anti-LRP4 antibody testing and promptly refer for electrophysiological studies to objectively assess neuromuscular transmission.
Repetitive nerve stimulation (RNS) and single-fiber EMG (SFEMG) are performed to demonstrate impaired neuromuscular transmission, providing definitive evidence for MG in all seronegative cases.
Alta DiagnoTech provides comprehensive in vitro diagnostic (IVD) solutions for myasthenia gravis (MG), including assay kits for anti-AChR, anti-MuSK, and anti-LRP4 antibodies utilizing technologies such as RIA, ELISA, CBA, and CLIA to support accurate serological subtyping and diagnosis. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| CQ-0087 | Biotin 3' End DNA Labeling Kit | Add To Cart |
| CA-00160 | Zika IgG/IgM and NS1 combo rapid assay kit | Add To Cart |
| IP-00187 | Influenza A+B rapid assay kit | Add To Cart |
| IP-00195 | Influenza H1N1 combo rapid assay kit | Add To Cart |
| CA-00156 | Dengue NS1 rapid assay kit | Add To Cart |
| CA-00162 | Typhoid IgG/IgM rapid assay kit | Add To Cart |
| IP-00177 | Cryptococcus antigen rapid assay kit | Add To Cart |
| CA-00183 | EBNA IgG rapid assay kit | Add To Cart |
| CA-00163 | Typhoid IgG/IgM rapid test kit | Add To Cart |
| CA-00150 | Astrovirus rapid assay kit | Add To Cart |
| CA-00151 | Norovirus, rotavirus, adenovirus and astrovirus combo rapid assay kit | Add To Cart |
| IP-00175 | Legionella pneumophila rapid assay kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |