- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Monkeypox Diagnostics: A Lab Guide to Accurate Detection & Testing Solutions
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Accurate and timely diagnosis of monkeypox virus (MPXV) is critical for effective patient management and outbreak control. This resource provides a detailed overview of current diagnostic methods, including gold-standard molecular tests, serological assays, and emerging rapid technologies. You'll also find essential guidance on optimizing testing workflows, ensuring quality control, and implementing best practices in your laboratory.
Monkeypox is a viral zoonotic disease caused by the monkeypox virus (MPXV), a member of the orthopoxvirus genus. The disease typically presents with fever, rash, and swollen lymph nodes, with symptoms lasting 2-4 weeks. While historically endemic to Central and West Africa, recent global outbreaks have highlighted its potential for human-to-human transmission through close contact, respiratory droplets, or contaminated materials. Most cases are mild, but severe illness can occur, particularly in immunocompromised individuals. Early and accurate diagnosis is essential to control transmission and ensure proper patient care.
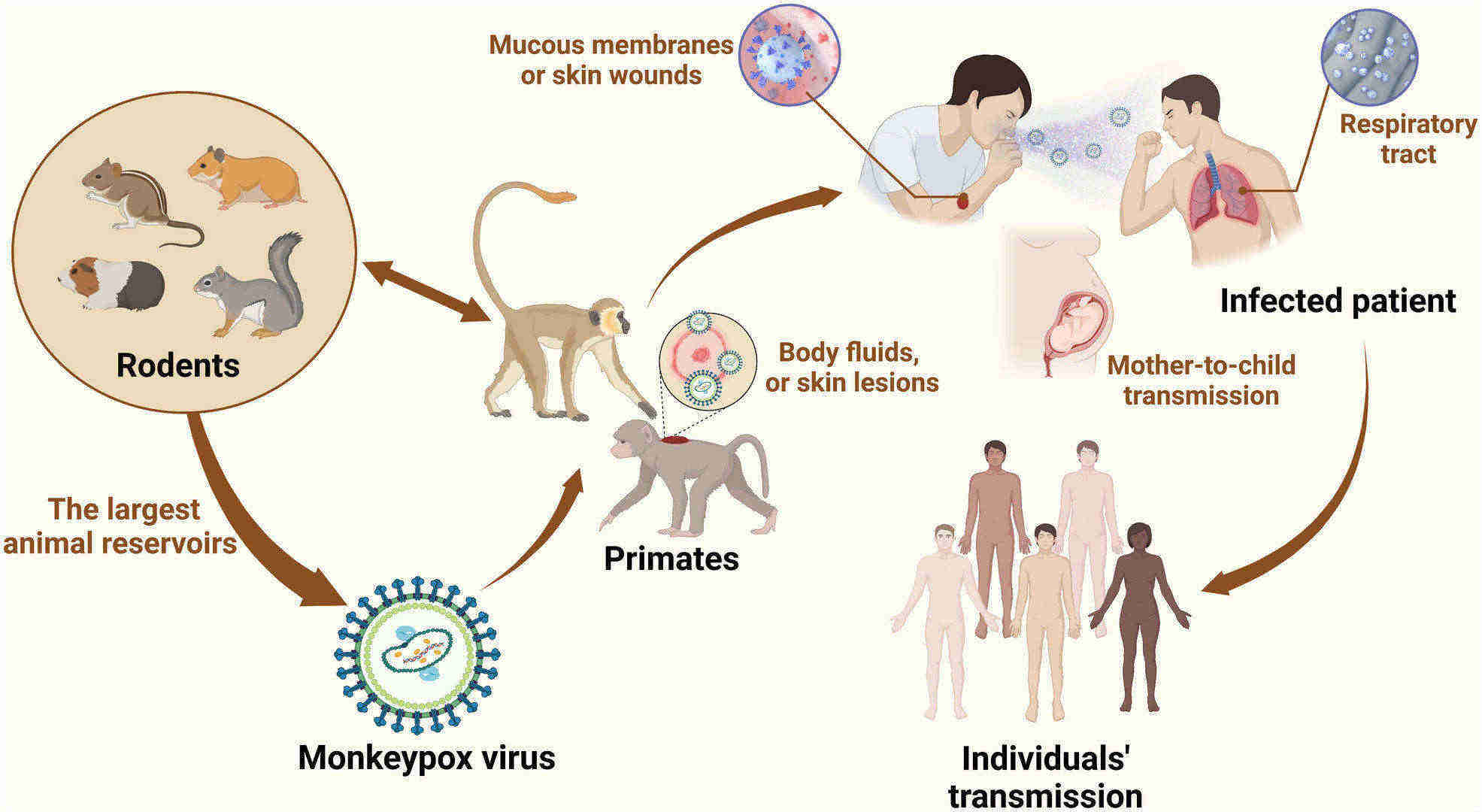 Fig.1 Schematic diagram of how monkeypox is transmitted. (Niu L, et al., 2023)
Fig.1 Schematic diagram of how monkeypox is transmitted. (Niu L, et al., 2023)
Accurate and timely laboratory testing is essential for confirming monkeypox virus (MPXV) infection, guiding clinical management, and implementing public health measures. Diagnostic methods can be broadly categorized into molecular techniques, serological assays, and antigen detection, each with distinct advantages and limitations.
Molecular techniques are the most reliable and sensitive methods for detecting monkeypox virus (MPXV), providing definitive diagnosis during active infection. These methods directly identify viral DNA, enabling early and accurate confirmation—critical for patient management and outbreak control.
The gold standard for MPXV diagnosis, qPCR amplifies and detects viral DNA (e.g., F3L, N3R genes) with high sensitivity and specificity. It delivers results within hours and is widely used in clinical labs following WHO/CDC protocols.
These methods, such as loop-mediated isothermal amplification and recombinase polymerase amplification, enable rapid and portable MPXV detection without the need for complex thermal cycling, making them well-suited for on-site or point-of-care testing (POCT).
Whole-genome or targeted sequencing confirms MPXV infection while providing strain-level data for outbreak tracking. Though slower and costlier than PCR, it is indispensable for identifying mutations, zoonotic origins, and antiviral resistance patterns.
Serological testing detects antibodies (IgM, IgG) produced in response to MPXV infection, providing retrospective diagnosis or seroprevalence data. These assays, including ELISA and neutralization tests, are particularly useful for epidemiological studies, vaccine response monitoring, and cases where PCR may yield false negatives. However, a key limitation is cross-reactivity with other orthopoxviruses (e.g., smallpox vaccine-induced immunity), which can complicate interpretation.
Antigen-based rapid diagnostic tests (RDTs) detect viral proteins in clinical samples (e.g., lesion swabs, serum) using lateral flow immunoassays, offering results in 15-30 minutes. These tests are ideal for point-of-care or resource-limited settings due to their portability and minimal equipment requirements. While less sensitive than PCR, they provide a practical screening tool during outbreaks when immediate triage is needed.
Reliable monkeypox virus (MPXV) diagnostics require rigorous optimization at every testing stage - from sample collection to final analysis. Implementing standardized protocols minimizes errors, enhances accuracy, and ensures consistent results across different laboratory settings. Three critical components form the foundation of high-quality MPXV testing: pre-analytical procedures, analytical validation, and ongoing quality control measures.


The future of monkeypox diagnostics is evolving toward faster, more accessible, and highly accurate testing solutions, driven by technological advancements and lessons from recent outbreaks. Emerging innovations include multiplex PCR panels for simultaneous detection of MPXV and other rash-causing pathogens, point-of-care tests for field deployment, and AI-powered interpretation tools to enhance diagnostic accuracy. Additionally, self-testing kits and wearable sensors are under exploration to improve early detection and outbreak containment.
Alta DiagnoTech offers a comprehensive portfolio of validated IVD products for accurate and efficient monkeypox detection, ensuring reliable performance across diverse laboratory settings. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| VT-QCY-0046 | Monkeypox Virus Nucleic Acid Test Kit (Fluorescent PCR Method) | Add To Cart |
| NATR-HMM-0018 | Monkeypox Virus Nucleic Acid Detection Reagent (Real-time PCR) | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |