- Home
- Resource
- Explore & Learn
- Digestive Enzyme Sensing for the Diagnosis and Monitoring of Pancreatitis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Pancreatitis, an inflammatory condition of the pancreas, poses significant diagnostic and therapeutic challenges. The pancreas, a critical organ in the digestive system, secretes enzymes such as amylase, trypsin, chymotrypsin, and lipase, which are essential for breaking down nutrients. When these enzymes are activated prematurely within the pancreas, they lead to self-digestion and inflammation, resulting in acute or chronic pancreatitis. Early and accurate detection of pancreatitis is crucial for effective management and to prevent complications. Traditional diagnostic methods, including imaging and blood tests, have limitations in terms of sensitivity, specificity, and accessibility. Therefore, the development of advanced in vitro diagnostic (IVD) techniques for detecting digestive enzymes has become a research priority. This article explores the latest advances in the detection and monitoring of pancreatitis through digestive enzyme sensing.
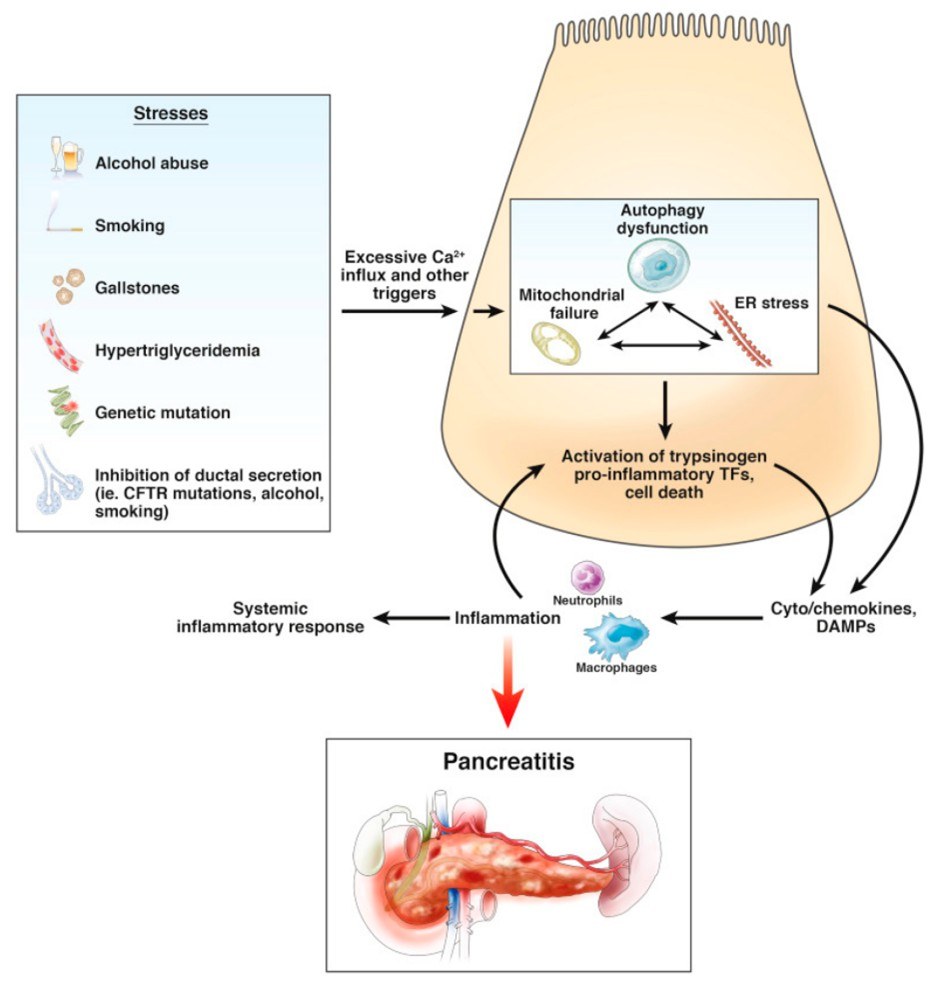 Fig.1 Summary of environmental stressors and genetic factors known to increase the risk for pancreatitis. (Yin J., et al., 2023)
Fig.1 Summary of environmental stressors and genetic factors known to increase the risk for pancreatitis. (Yin J., et al., 2023)
Enzymatic Pathways and Pancreatitis
The pancreas secretes a suite of digestive enzymes that play pivotal roles in the digestion of carbohydrates, proteins, and fats. Amylase hydrolyzes carbohydrates, while proteases like trypsin and chymotrypsin break down proteins. Lipase is responsible for the digestion of fats. In pancreatitis, the premature activation of these enzymes within the pancreatic acinar cells leads to tissue damage and inflammation. The severity of pancreatitis can vary from mild, self-limiting episodes to severe, life-threatening conditions.
Clinical Biomarkers for Pancreatitis
Elevated levels of pancreatic enzymes in the blood and urine are hallmark indicators of pancreatitis. Serum amylase and lipase levels, for instance, are commonly used as diagnostic markers for acute pancreatitis (AP). However, these markers lack specificity, as they can be elevated in other conditions such as renal failure and gastrointestinal perforation. Therefore, the development of more accurate and specific biomarkers is essential for improving the diagnosis of pancreatitis.
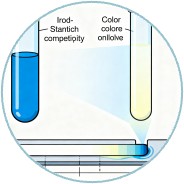
Colorimetric sensors offer a simple and cost-effective method for detecting digestive enzymes. These sensors rely on the color change of a substrate upon enzymatic hydrolysis. For example, the iodine-starch complex forms a blue color that disappears as amylase hydrolyzes starch. This principle has been applied in paper-based sensors, where the distance a colored solution travels on pH test paper indicates enzyme activity. Such sensors are particularly useful for point-of-care testing (POCT) in resource-limited settings.
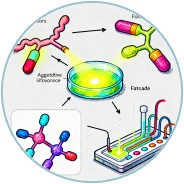
Fluorescence-based sensors provide high sensitivity and specificity for enzyme detection. These sensors utilize fluorescent probes that emit light upon interaction with the target enzyme or its hydrolysis products. For instance, aggregation-induced emission (AIE) probes have been designed to detect lipase activity by fluorescing upon interaction with fatty acids released during lipolysis. Fluorescence sensors can be integrated into microfluidic devices for high-throughput screening.

SPR sensors detect changes in the refractive index of a metal surface upon interaction with target molecules. By immobilizing enzyme-specific substrates on the metal surface, SPR sensors can detect enzyme activity through changes in the resonance angle. This technique has been applied to detect trypsin and other proteases with high sensitivity. SPR sensors offer real-time monitoring and can be used for kinetic studies of enzyme reactions.
Enzymatic hydrolysis can alter the viscosity of a solution, which can be detected using mechanical sensors. For example, the viscosity change caused by amylase hydrolysis has been used to develop paper-based sensors for POCT. These sensors are simple and inexpensive, making them suitable for use in low-resource settings.
Acoustic wave sensors, such as thickness-shear mode (TSM) resonators, detect changes in mechanical resonance frequency caused by enzymatic reactions. These sensors have been applied to detect proteases like trypsin and chymotrypsin with high accuracy. Acoustic wave sensors offer non-invasive and label-free detection, making them attractive for clinical applications.
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| IEC-HMM-0013 | S-Adenosine Homocysteine Hydrolase, SAHH | Add To Cart |
| IEC-HMM-0045 | Uracil-DNA Glycosylase (UNG), Heat-labile | Add To Cart |
| IEC-HMM-0005 | Pyruvate Kinase, PK | Add To Cart |
| IEC-HMM-0034 | Proteinase K | Add To Cart |
| IEC-HMM-0033 | Cystathionine β-synthase, CBS | Add To Cart |
| IEC-HMM-0035 | Proteinase K Liquid (20 mg/mL) | Add To Cart |
| IEC-HMM-0012 | Homocysteine Methyltransferase, HMT | Add To Cart |
| IEC-HMM-0015 | S-adenosylmethionine Synthetase | Add To Cart |
| IEC-HMM-0027 | Mn-Superoxide Dismutase | Add To Cart |
| IEC-HMM-0041 | Uracil-DNA Glycosylase (UNG), E.coli | Add To Cart |
| IEC-HMM-0023 | Glutathione S-transferase | Add To Cart |
| IEC-HMM-0001 | AcyI-CoA Oxidase, ACO | Add To Cart |
| IEC-HMM-0031 | Malatedehydrogenase, MDH | Add To Cart |
| IEC-HMM-0014 | N-Acetylneuraminic Acid Aldolase, NAL | Add To Cart |
| IEC-HMM-0017 | Neuraminidase, NRH | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |