- Home
- Resource
- Disease Diagnosis
- Genetic Diseases
- Advancing Sickle Cell Disease (SCD) Diagnosis: Cutting-Edge Detection Technologies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Early and accurate diagnosis is critical for timely intervention of sickle cell disease (SCD). This resource provides a comprehensive overview of SCD diagnostic approaches, spanning established laboratory methods such as HPLC and electrophoresis, innovative point-of-care diagnostic solutions such as lateral flow assays and sensor-based devices, and advanced genetic testing technologies.
Sickle cell disease (SCD) is a life-threatening inherited hemoglobin disorder caused by a mutation in the HBB gene, resulting in abnormal hemoglobin S (HbS) that distorts red blood cells into a sickle shape. This leads to chronic hemolytic anemia, vaso-occlusive crises, and progressive multi-organ damage. With over 300,000 newborns affected annually worldwide, SCD demands early diagnosis through newborn screening (NBS) programs, which enable critical early interventions like penicillin prophylaxis and vaccination.
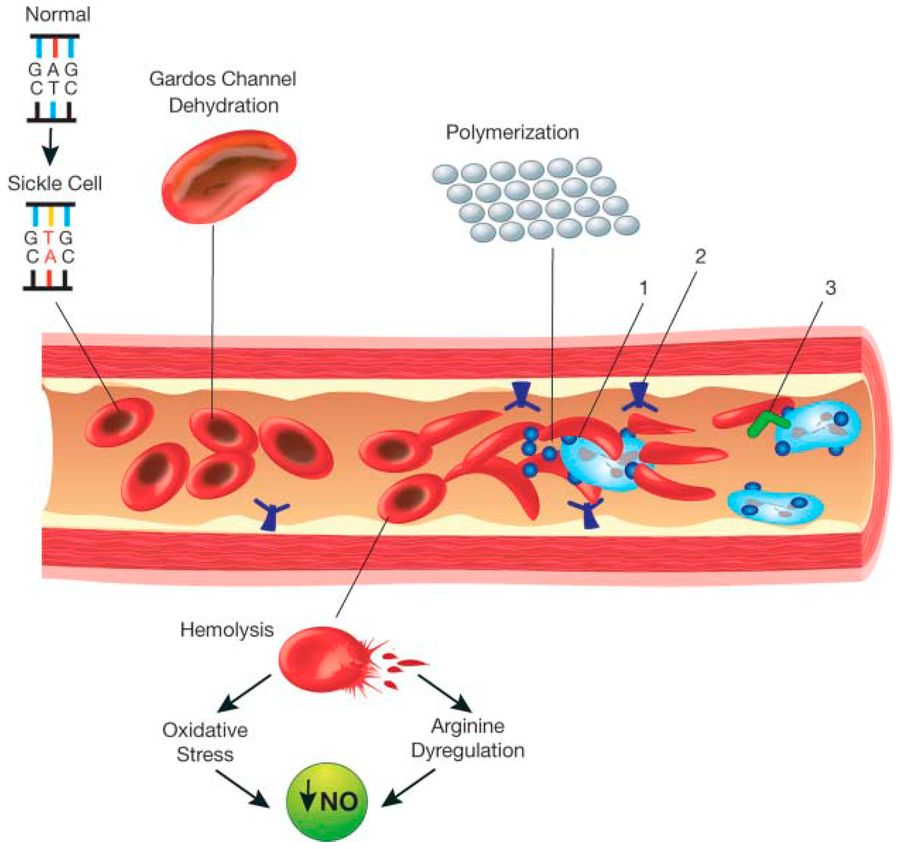 Fig.1 Schematic representation of the pathophysiology (in part) of sickle cell anemia. (Inusa B P D, et al., 2019)
Fig.1 Schematic representation of the pathophysiology (in part) of sickle cell anemia. (Inusa B P D, et al., 2019)
Accurate and timely diagnosis of sickle cell disease (SCD) is essential to prevent life-threatening complications and initiate life-saving interventions. While newborn screening programs have significantly improved early detection in many countries, diagnostic challenges persist, particularly in resource-limited settings and for atypical presentations. Current diagnostic approaches combine simple screening tests with advanced confirmatory methods to detect hemoglobin abnormalities. Here we outline the key techniques used in modern SCD diagnosis:
Complete Blood Count (CBC)
The CBC reveals characteristic anemia (Hb 6-9 g/dL) with elevated reticulocytes (3-15%) due to chronic hemolysis. While nonspecific, it provides the first clue to possible SCD, especially when combined with clinical symptoms.

Peripheral Blood Smear
Microscopic examination shows sickle cells, target cells, and Howell-Jolly bodies (indicating functional asplenia). Though diagnostic when positive, it may miss cases without active sickling, requiring confirmatory testing.
Solubility Sickling Test
This inexpensive bedside test detects HbS polymerization under low oxygen conditions. While rapid (results in 10-15 minutes), it cannot distinguish between sickle cell trait (HbAS) and disease (HbSS), nor identify other hemoglobin variants.
Hemoglobin Electrophoresis
The traditional gold standard separates hemoglobin variants by charge at alkaline (pH 8.6) and acid (pH 6.2) conditions. It reliably identifies HbS, HbC, and HbF but requires technical expertise and has slower turnaround than newer methods.
Isoelectric Focusing (IEF)
This high-resolution technique separates hemoglobins by isoelectric point, offering superior sensitivity to electrophoresis for detecting low-level abnormal hemoglobins. It's commonly used in newborn screening programs.
High Performance Liquid Chromatography (HPLC)
Now the preferred method in most labs, HPLC provides automated, quantitative hemoglobin analysis with excellent precision. It can detect and quantify all clinically relevant hemoglobin variants simultaneously, making it ideal for both screening and confirmation.
While hemoglobin-based tests detect abnormal hemoglobin variants, genetic testing provides definitive diagnosis by identifying the underlying HBB gene mutation (Glu6Val). This is particularly valuable for atypical cases, prenatal/carrier screening, and differentiating sickle cell disease (SCD) from other hemoglobinopathies. Three key molecular techniques enable precise SCD genetic diagnosis:
Polymerase Chain Reaction (PCR) methods like ARMS-PCR rapidly amplify and detect the sickle mutation (Glu6Val) in the HBB gene. Ideal for high-throughput newborn screening confirmation, these tests deliver results in hours with minimal DNA requirements, though they only target known mutations.

RFLP uses restriction enzymes (e.g., DdeI) to cleave DNA at the sickle mutation site, producing distinct electrophoretic patterns. This gold-standard method provides absolute mutation confirmation but requires gel electrophoresis, making it slower than PCR-based alternatives.

Microarrays simultaneously screen for multiple hemoglobin mutations, while next-generation sequencing (NGS) identifies rare variants and compound heterozygotes through whole HBB gene analysis. These advanced tools resolve complex cases but remain costlier than targeted methods, with NGS offering future potential for severity prediction.
Traditional sickle cell disease (SCD) diagnostics like HPLC and electrophoresis, while accurate, often require sophisticated lab infrastructure and trained personnel. Emerging point-of-care (POC) and rapid screening technologies are revolutionizing SCD detection, particularly in low-resource settings, by offering affordable, portable, and user-friendly alternatives without compromising diagnostic reliability.
A rapid diagnostic method utilizing antibody-coated strips to detect hemoglobin S (HbS) through capillary flow. This technique provides visual results within minutes without requiring specialized equipment, making it suitable for point-of-care settings. While highly accessible, it typically offers qualitative rather than quantitative results.
A simplified diagnostic approach where chemically treated paper identifies HbS polymerization. This method is cost-effective, portable, and stable under various environmental conditions, though it lacks the ability to distinguish between carrier and disease states.
Advanced diagnostic systems employing microfluidic, electrochemical, or optical mechanisms to analyze hemoglobin variants. These technologies enable precise HbS detection with potential integration into portable or automated platforms, combining accuracy with adaptability for diverse healthcare settings.
The future of sickle cell disease (SCD) diagnostics is moving toward decentralized, precision solutions that integrate artificial intelligence, portable technologies, and advanced genomics to enable faster, more convenient, and more personalized testing. Emerging innovations such as wearable biosensors and AI-driven interpretation tools aim to move screening from resource-rich laboratories to point-of-care and home settings. These advances will not only enhance early diagnosis, but also facilitate disease monitoring and personalized treatment strategies.
At Alta DiagnoTech, we offer comprehensive in vitro diagnostic (IVD) solutions for sickle cell disease (SCD), encompassing test kits, instruments, and consumables to facilitate accurate and efficient testing. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |