- Home
- Resource
- Explore & Learn
- Validation of a Saliva-Based MMP-1 Rapid Test for Detecting Oral Cavity Cancer in Clinical Settings
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Oral cancer remains one of the most deadly cancers worldwide, with high mortality rates due to late-stage diagnoses. Yet, recent advances in in vitro diagnostics (IVD) suggest that a simple, non-invasive test utilizing saliva could transform the way oral cancer is detected and monitored. This method hinges on the detection of specific biomarkers in saliva, a natural fluid that offers an array of diagnostic opportunities. Matrix metalloproteinase-1 (MMP-1) has emerged as one of the most promising biomarkers for oral squamous cell carcinoma (OSCC), the most common form of oral cancer. The development of rapid strip tests (RST) to detect MMP-1 levels in saliva promises to be a game-changer for early detection, potentially saving countless lives through more accessible and timely diagnoses.
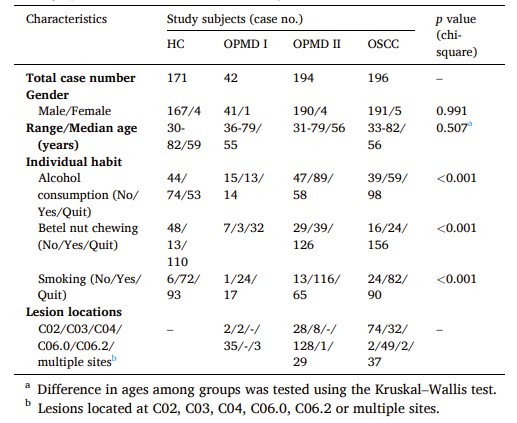 Fig.1 Demographics of clinical cases in this study. (Chu L. J., et al., 2024)
Fig.1 Demographics of clinical cases in this study. (Chu L. J., et al., 2024)
Oral cancer, including OSCC, is among the leading causes of cancer-related deaths worldwide. In 2020 alone, it was estimated that more than 657,000 new cases of oral cancer were diagnosed globally, resulting in approximately 330,000 deaths. The prognosis for patients diagnosed with oral cancer is heavily dependent on the stage at which the cancer is identified. Early-stage OSCC can be treated more effectively, with much higher survival rates. However, diagnosing oral cancer at an early stage remains a significant challenge.
Symptoms of oral cancer often do not present until the disease has reached an advanced stage. Many patients do not exhibit obvious signs until the cancer has already spread to lymph nodes or other areas, making it more difficult to treat effectively. The traditional method of diagnosing OSCC involves tissue biopsy, which is invasive, time-consuming, and requires skilled clinicians to accurately select the biopsy sites. This process is costly and may cause delays in diagnosis, with over half of OSCC cases detected at an advanced stage.
Saliva has long been recognized as a potential source of diagnostic biomarkers due to its non-invasive nature and its ability to reflect the body's physiological status. It contains a wide array of proteins, enzymes, hormones, and metabolites that mirror the body's internal processes. The potential of saliva as a diagnostic tool has become increasingly apparent, particularly for oral cancer, where biomarkers present in the saliva can indicate the early stages of the disease.
Recent research has focused on identifying specific salivary biomarkers for OSCC. These biomarkers include proteins, RNA molecules, and metabolites that exhibit abnormal expression in cancerous cells compared to healthy tissues. One of the most promising biomarkers identified for OSCC detection is matrix metalloproteinase-1 (MMP-1), an enzyme involved in the breakdown of extracellular matrix components. MMP-1 is upregulated in several cancers, including OSCC, and its elevated levels in saliva have been strongly correlated with the presence of oral cancer.

Matrix metalloproteinases (MMPs) are a family of enzymes that play a critical role in tissue remodeling, wound healing, and inflammation. Among these enzymes, MMP-1, also known as interstitial collagenase, is particularly significant in cancer biology. MMP-1 facilitates the degradation of collagen in the extracellular matrix, a process that is essential for tumor invasion and metastasis. In the case of OSCC, elevated MMP-1 levels contribute to the breakdown of oral tissues, enabling cancer cells to invade deeper into the tissue layers and spread to other regions.
In clinical studies, MMP-1 has been found to be consistently upregulated in the saliva of patients with OSCC compared to healthy individuals or those with oral potentially malignant disorders (OPMDs). The concentration of MMP-1 in saliva is also correlated with the size and progression of the tumor. Higher levels of MMP-1 are associated with more advanced stages of OSCC, making it an ideal candidate for use as a biomarker for both early detection and monitoring of disease progression.
Although enzyme-linked immunosorbent assays (ELISA) have been used to measure MMP-1 levels in saliva, they are time-consuming and require laboratory equipment. In contrast, the rapid strip test (RST) is a simple, on-site diagnostic tool that offers significant advantages over traditional methods. The RST can provide results within minutes, making it ideal for point-of-care testing. It also requires minimal equipment and is easy to use, reducing both the cost and complexity of the testing process.
The MMP-1 RST works by detecting the presence of MMP-1 in saliva through a colorimetric reaction. When saliva samples are mixed with the test reagent, a visible line appears on the test strip if MMP-1 is present in sufficient quantities. The intensity of the test line correlates with the level of MMP-1 in the sample, providing a quantitative measure of the biomarker. This approach makes the MMP-1 RST a promising tool for oral cancer diagnosis in settings such as pharmacies, dental clinics, and even in rural or resource-limited environments.

To assess the effectiveness of the MMP-1 RST, a clinical study was conducted involving 603 participants, including healthy controls, patients with OPMDs, and OSCC patients. The study compared the performance of the MMP-1 RST with the traditional ELISA method. The results showed that both methods performed similarly in terms of sensitivity, specificity, and accuracy, confirming the potential of the RST as a reliable diagnostic tool for OSCC.
These results demonstrate that the MMP-1 RST can effectively differentiate between OSCC patients and healthy individuals or those with OPMDs, providing evidence for its potential use in clinical practice.
Early detection is crucial for improving the prognosis of OSCC patients. As the MMP-1 RST can detect elevated levels of MMP-1 in saliva, it has the potential to identify OSCC at an early stage, when treatment options are most effective. The study demonstrated that the RST could accurately distinguish between OSCC patients at different stages of cancer, including early-stage (stage I) and advanced-stage (stage IV) disease.
By identifying OSCC in its early stages, the RST could significantly improve survival rates and reduce the need for aggressive treatments. Early-stage detection also allows for more targeted therapies, which can minimize side effects and improve quality of life for patients.
The MMP-1 RST has shown promise in distinguishing OSCC at various stages of the disease. In the clinical study, median MMP-1 levels were found to increase with disease progression, from stage I (13.2 RLU) to stage IV (45.7 RLU). This correlation between MMP-1 levels and cancer stage suggests that the RST could not only aid in early detection but also help monitor disease progression over time.
The ability to differentiate between early and late-stage OSCC using the MMP-1 RST could be invaluable for clinicians in assessing the severity of the disease and determining the most appropriate course of treatment.
The introduction of the MMP-1 RST represents a significant step forward in oral cancer screening. By providing a simple, affordable, and reliable method for detecting OSCC, the RST has the potential to become a key tool in public health initiatives aimed at reducing the burden of oral cancer.
One of the major barriers to early oral cancer detection is access to diagnostic resources. In many parts of the world, especially in rural areas, individuals may not have access to specialized medical facilities where biopsies and other diagnostic tests can be performed. The MMP-1 RST offers a solution to this problem by allowing for on-site, rapid testing that can be administered in a variety of healthcare settings. This accessibility could lead to widespread adoption of the test, ensuring that more individuals are screened for oral cancer and that those who need treatment receive it in a timely manner.
The ease of use and low cost of the MMP-1 RST make it an ideal addition to routine dental checkups. Dentists and oral health professionals could use the test as part of regular screenings for individuals at risk of developing oral cancer. In combination with visual inspections, the MMP-1 RST could provide a more comprehensive approach to oral cancer detection, helping to identify suspicious lesions before they progress to malignant stages.
The development of the MMP-1 rapid strip test represents a groundbreaking advancement in the early detection of oral cancer. By leveraging the power of saliva and identifying a reliable biomarker, this non-invasive test provides a simple, cost-effective, and accessible method for diagnosing OSCC. With the potential to detect cancer at its earliest stages, the MMP-1 RST could save countless lives by facilitating timely interventions and reducing the burden of oral cancer globally. As this diagnostic tool becomes more widely available, it has the potential to revolutionize oral cancer screening and significantly improve patient outcomes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |