- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
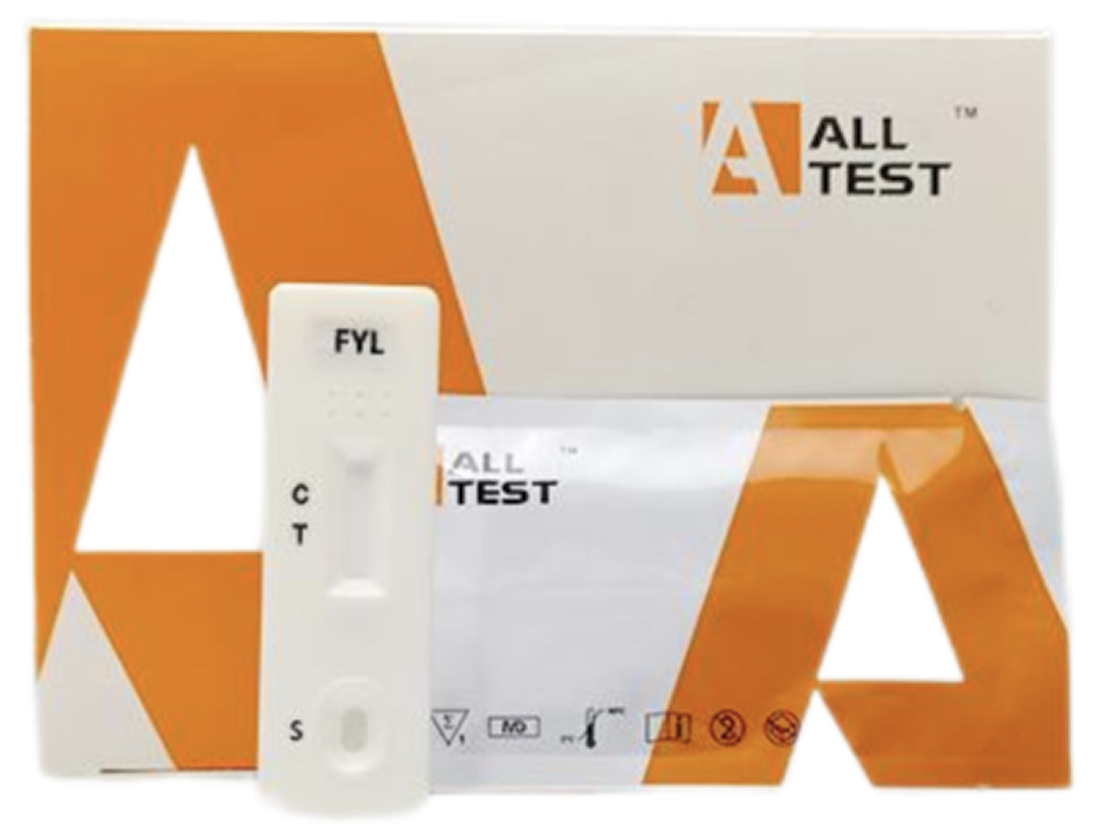
| Product Name | Fentanyl Urine Test Cassette (CLIA Waived) |
| Catalog No. | ID-HYW-0009 |
| Description | This fentanyl test cassette can be used for different professions such as clinics, physician’s offices, or urgent care facilities by testers with no required additional qualification. The test’s easy format enables healthcare professionals to perform the test and provide immediate results at the locations of need. The CLIA-waived designation reduces the level of required training allowing greater confidence in programmatic performance and ease in drug screening initiatives. |
| Species Characteristics | Human |
| Detection Principle | Immunochromatographic method (ICT) |
| Specificity | Fentanyl (FYL) |
| Cut Off | 1 ng/mL |
| Sample Type | Urine |
| Testing Time | 5 minutes |
| Material | Cassette |
| Application | The fentanyl rapid test (urine) is an immunoassay intended for the qualitative detection of fentanyl in human urine at a cutoff concentration of 1.0 ng/mL. This in vitro diagnostic device is CLIA waived. |
| Storage | Store at 2-30°C |
| Operating Temperature | Operate at 4-30°C |
| Transportation Condition | Transportation at room temperature |
| Shelf Life | 2 years |
For in vitro diagnostic use.
|
There is no product in your cart. |