- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company




Cat.No: GH-DQL-00200 Datasheet
Specification
Quantities
Specification
Quantities
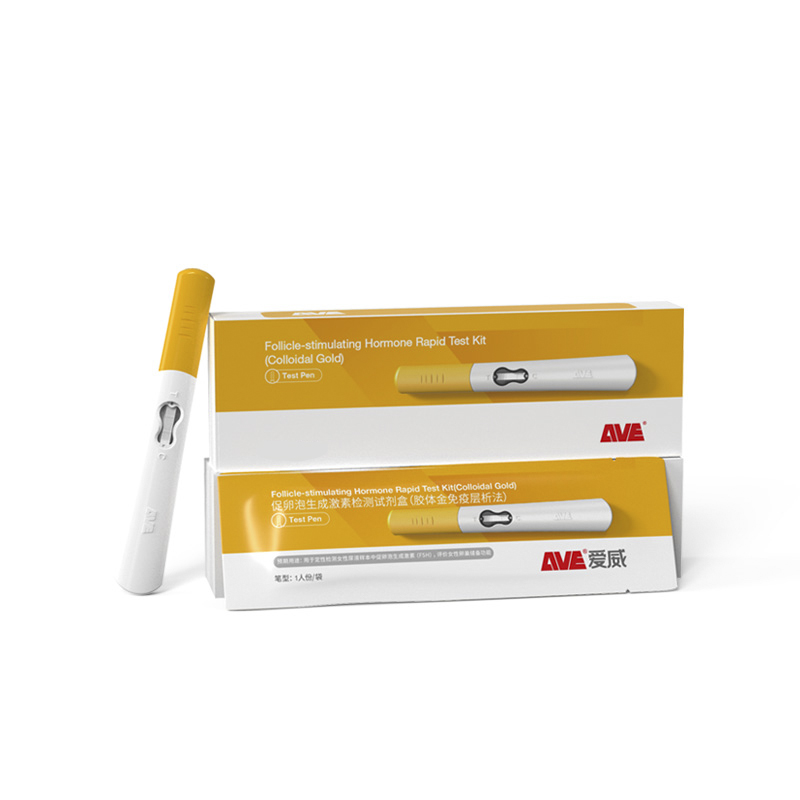
| Product Name | Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold) |
| Catalog No. | GH-DQL-00200 |
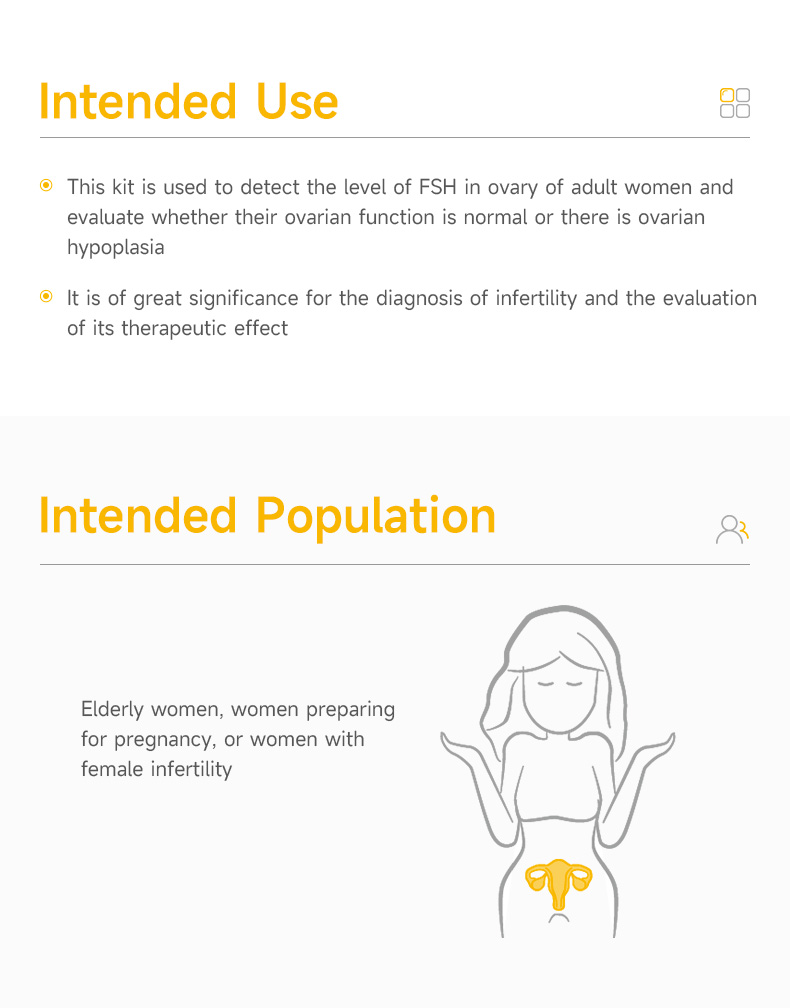
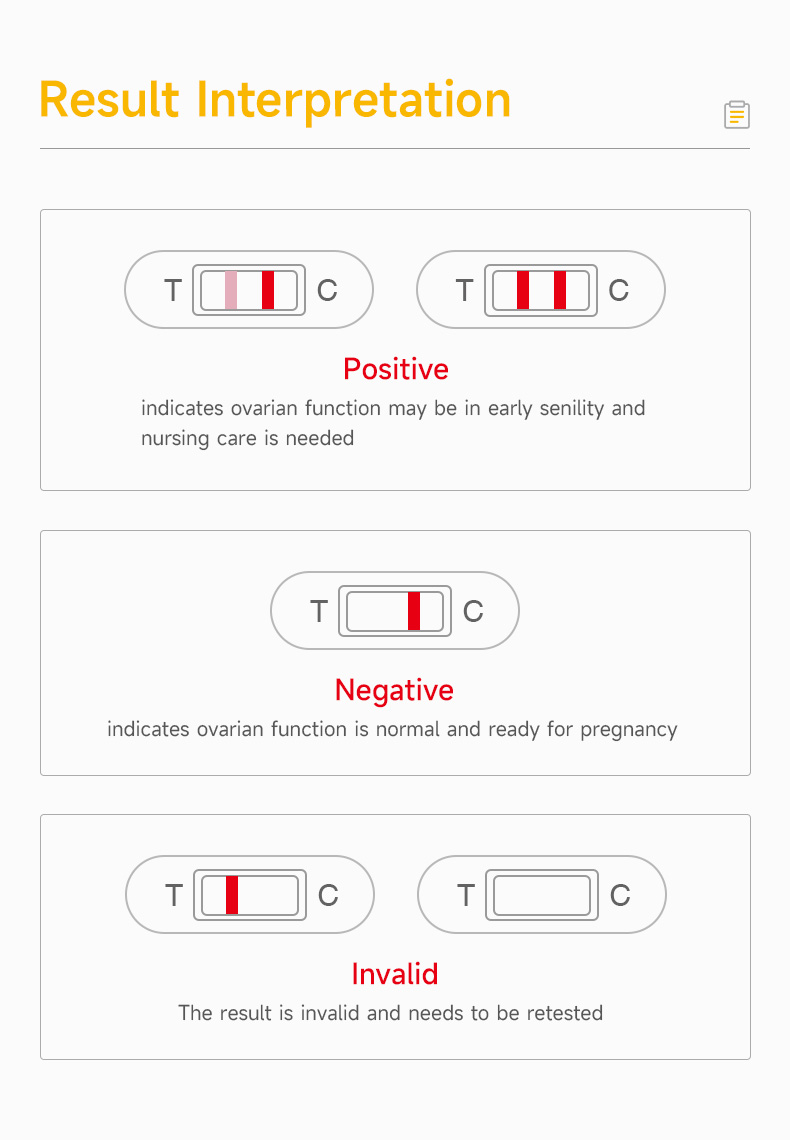
| Description | This test kit is used for the qualitative detection of follicle-stimulating hormone (FSH) in urine, to help assess ovarian function. |
| Features | Urine collection for testing, convenient sampling, non-invasive, and painless. Highly specific antigen-antibody binding for accurate and reliable results. Simple operation, fast detection. |
| Certification | FDA |
| Main Compositions | Detection area (coated with mouse anti-FSH monoclonal antibody), quality control zone (coated with sheep anti-chicken IgY polyclonal antibody), colloidal gold conjugate pad (coated with mouse anti-human FSH monoclonal antibody and chicken IgY polyclonal antibody), nitrocellulose membrane, and plastic support. |
| Detection Principle | This test kit adopts the bi-antibody sandwich method. |
| Application | The reagent is used to test the level of follicle-stimulating hormone (FSH) in female urine samples. |
| Storage Conditions | 2°C~40°C |
| Valid Period | 24 months |
| Sample Type | Urine |
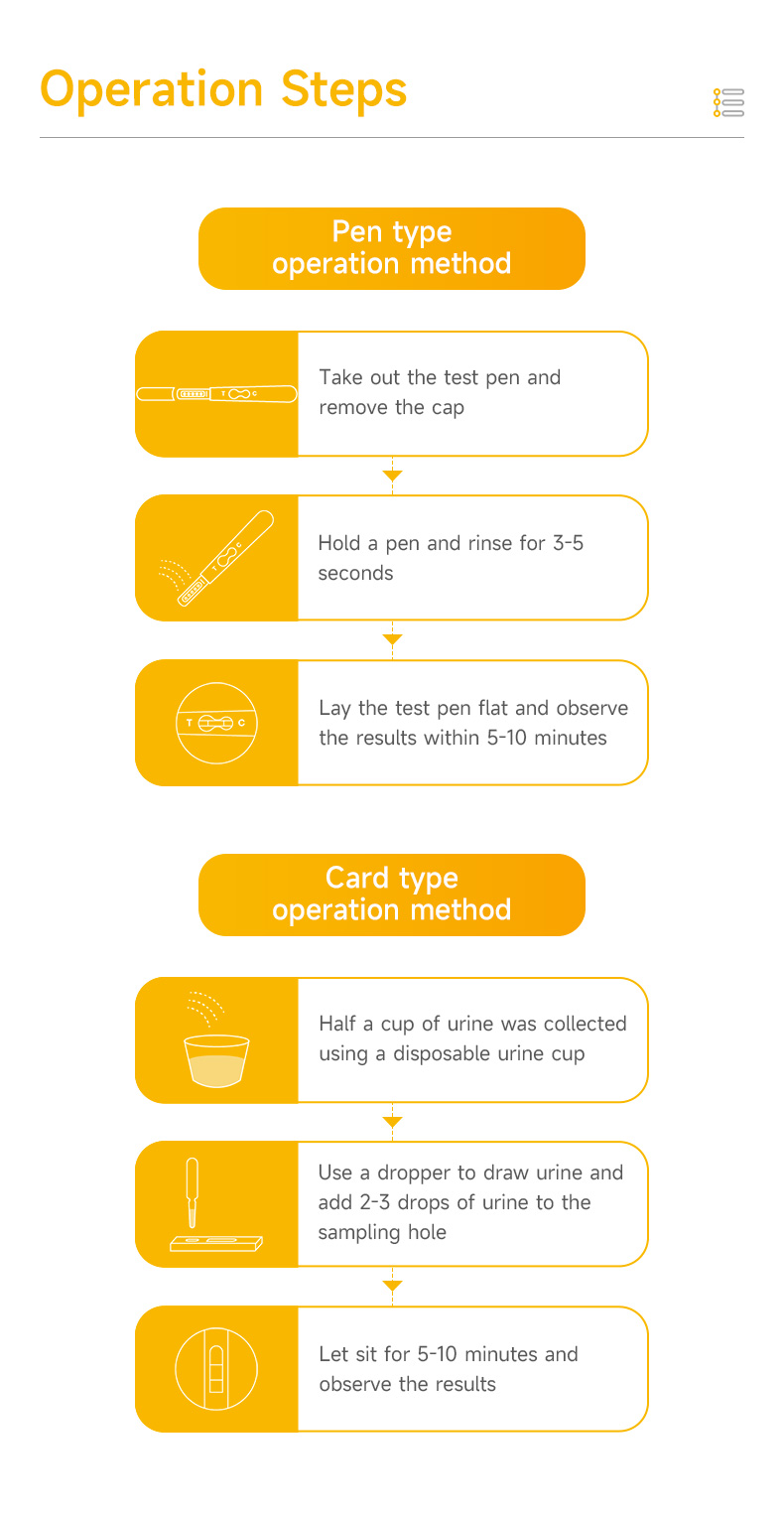
| Test Time | 5~10 minutes |
| Test Temperature | 10°C |
| Test Humidity | ≤80% |
| Reference Range | When FSH concentration is ≥25 mIU/mL, the result is positive. |

For in vitro diagnostic use.
|
There is no product in your cart. |