- Home
- Resource
- Explore & Learn
- Unveiling the Impact of Maternal Bisphenol A Exposure on Offspring Health
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Bisphenol A (BPA), a widely recognized endocrine-disrupting chemical (EDC), has come under increasing scrutiny due to its ubiquitous presence in consumer products and its potential to cause adverse health effects. Commonly found in plastics, food and beverage containers, medical devices, and thermal paper, BPA can readily leach into food and beverages, thereby exposing humans to its potentially harmful effects. Of particular concern is its ability to transfer from mother to child during pregnancy and lactation, which raises significant questions about the long-term health consequences for offspring. This article seeks to provide a thorough examination of the impact of maternal BPA exposure on the health of offspring, with a focus on metabolic, hormonal, and behavioral outcomes.
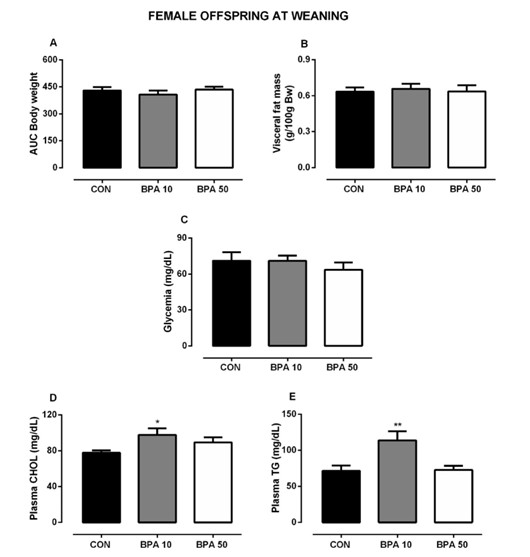 Fig.1 Effects of oral BPA administration during gestation and breastfeeding on biometric and plasma parameters of the female rat offspring at weaning. (Silva B. S., et al., 2019)
Fig.1 Effects of oral BPA administration during gestation and breastfeeding on biometric and plasma parameters of the female rat offspring at weaning. (Silva B. S., et al., 2019)
Furthermore, BPA exposure has been associated with impaired glucose tolerance and reduced insulin sensitivity, which are key hallmarks of metabolic syndrome. These metabolic disruptions may arise from BPA's ability to interfere with critical biological pathways, including insulin signaling and lipid biosynthesis. By disrupting the normal functioning of these pathways, BPA can impair the body's ability to effectively process glucose and lipids, leading to a cascade of metabolic imbalances. Over time, these imbalances can predispose individuals to the development of obesity, type 2 diabetes, and other metabolic disorders.
The mechanisms underlying these metabolic disruptions are complex and multifaceted. BPA's endocrine-disrupting properties allow it to interfere with the normal functioning of hormones that regulate metabolism, such as insulin and adipokines. Additionally, BPA can influence the expression of genes involved in lipid metabolism and energy homeostasis, further contributing to the observed metabolic disturbances. For instance, BPA has been shown to modulate the activity of key enzymes involved in lipid synthesis and breakdown, leading to an imbalance in lipid metabolism.
Moreover, BPA exposure during critical developmental windows can have lasting epigenetic effects, altering the expression of genes involved in metabolic regulation. These epigenetic changes can persist into adulthood, influencing metabolic function and increasing susceptibility to metabolic disorders. The cumulative impact of these disruptions underscores the significant threat that maternal BPA exposure poses to the long-term metabolic health of offspring.
Sex Steroid Hormones
Bisphenol A (BPA) is a well-documented xenoestrogen, meaning it has the capacity to bind to estrogen receptors and mimic or disrupt the actions of natural estrogens. This characteristic has far-reaching implications for the levels of sex steroid hormones in offspring exposed to BPA during critical developmental periods. Extensive research has demonstrated that maternal exposure to BPA can significantly elevate plasma levels of progesterone, testosterone, and estradiol in both male and female offspring at the time of weaning. However, these hormonal alterations are often highly specific to sex and age, indicating complex and nuanced effects on the developing endocrine system.
For example, female offspring exposed to BPA during gestation and lactation may exhibit normal estrous cycles, suggesting that the immediate reproductive functions are not severely compromised. In contrast, male offspring often display reduced testosterone levels as they reach adulthood, pointing to a potential disruption in the hypothalamic-pituitary-gonadal (HPG) axis. This axis is crucial for regulating reproductive function and maintaining normal levels of sex hormones, and its disruption can have long-term consequences for fertility and overall reproductive health. The sex-specific nature of these effects underscores the complexity of BPA's impact on the endocrine system and highlights the need for targeted research to fully understand its mechanisms of action.
Thyroid Hormones
Thyroid hormones are essential for a wide range of physiological processes, particularly during fetal and neonatal development. They play a critical role in regulating metabolism, neurological development, and overall growth. Consequently, any disruption to thyroid hormone levels can have profound and lasting consequences on an individual's health.
Recent studies have shown that maternal BPA exposure can significantly alter thyroid hormone levels in offspring. Specifically, both male and female offspring exposed to BPA during gestation and lactation have been found to exhibit decreased plasma concentrations of triiodothyronine (T3) and thyroxine (T4). These reductions in thyroid hormone levels can impair neurological development, potentially leading to cognitive deficits and behavioral abnormalities later in life. Additionally, the disruption of thyroid hormone regulation can affect metabolic processes, contributing to the increased risk of metabolic disorders observed in BPA-exposed individuals.
Given the critical role of thyroid hormones in early development, these findings highlight the potential for BPA to have significant adverse effects on the long-term health and well-being of offspring. Understanding the mechanisms by which BPA disrupts thyroid hormone regulation is essential for developing strategies to mitigate these risks and protect vulnerable populations.
Stress Hormones
The hypothalamic-pituitary-adrenal (HPA) axis is a central regulatory system that governs the body's response to stress. It plays a crucial role in maintaining homeostasis and ensuring that the body can effectively respond to both acute and chronic stressors. However, emerging evidence suggests that BPA exposure can significantly impact the functioning of the HPA axis, potentially leading to long-term alterations in stress reactivity and overall stress management.
Animal studies have reported altered levels of corticosterone, a primary stress hormone, in offspring exposed to BPA during critical developmental periods. Some studies have observed increased corticosterone levels, indicating heightened stress reactivity and a potential for exaggerated responses to stress. Conversely, other studies have reported decreased corticosterone levels, suggesting suppression of the HPA axis and a reduced ability to mount an appropriate stress response. These discrepancies highlight the complexity of BPA's effects on the HPA axis and may arise from differences in experimental design, including the timing and duration of BPA exposure, as well as the specific animal models used.
Understanding the precise mechanisms by which BPA affects the HPA axis is crucial for elucidating its broader impact on stress-related health outcomes. Chronic alterations in stress hormone levels can have far-reaching consequences, including increased susceptibility to anxiety, depression, and other stress-related disorders. Additionally, dysregulation of the HPA axis can impact immune function, metabolic processes, and overall health resilience. Further research is needed to clarify these mechanisms and to develop strategies to mitigate the potential adverse effects of BPA on stress regulation.

One of the key mechanisms by which BPA exerts its effects is through epigenetic modifications, including DNA methylation and histone acetylation. These changes can lead to altered gene expression patterns, particularly in genes involved in metabolic regulation, hormonal signaling, and neurodevelopment. For instance, BPA exposure has been shown to induce DNA methylation changes in genes encoding for adipogenic enzymes, leading to increased fat accumulation.
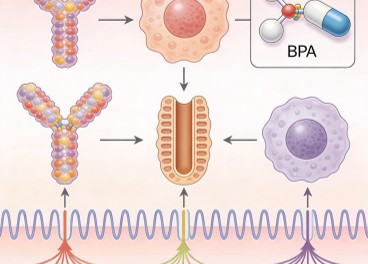
BPA's ability to bind to various hormone receptors, including estrogen, androgen, thyroid, and glucocorticoid receptors, allows it to interfere with multiple signaling pathways. By mimicking or antagonizing the actions of endogenous hormones, BPA can disrupt normal physiological processes, leading to metabolic, hormonal, and behavioral disturbances. For example, BPA's binding to estrogen receptors can activate or inhibit estrogen-responsive genes, altering cellular responses and tissue development.
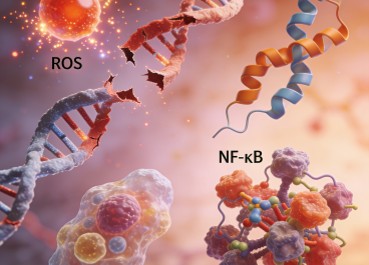
BPA exposure has also been linked to increased oxidative stress and inflammation, both of which play key roles in the pathogenesis of various diseases. Oxidative stress can damage cellular components, including DNA, proteins, and lipids, leading to cellular dysfunction and apoptosis. Inflammation, on the other hand, can disrupt tissue homeostasis and contribute to the development of chronic diseases. BPA-induced oxidative stress and inflammation may be mediated by the activation of nuclear factor-kappa B (NF-κB) and other pro-inflammatory signaling pathways.
The evidence presented in this article underscores the profound and lasting impact of maternal BPA exposure on offspring health. From metabolic disruptions and hormonal imbalances to behavioral and neurodevelopmental effects, BPA's ability to interfere with multiple physiological systems poses a significant threat to public health. To mitigate these risks, it is imperative to reduce BPA exposure through regulatory measures, such as banning BPA in food and beverage containers and promoting the use of safer alternatives.
Future research should focus on elucidating the precise mechanisms by which BPA exerts its effects, particularly at the molecular and epigenetic levels. Longitudinal studies in humans, along with mechanistic studies in animal models, will be crucial for understanding the long-term health consequences of BPA exposure and developing effective strategies for prevention and intervention. By taking proactive measures to address this public health challenge, we can protect future generations from the adverse effects of BPA and other endocrine-disrupting chemicals.
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| IH-HYW-0002 | hCG Pregnancy Test Cassette | Add To Cart |
| IH-HYW-0003 | hCG Pregnancy Test Midstream | Add To Cart |
| IH-HYW-0007 | ShinetelI™ Digital Pregnancy Test | Add To Cart |
| IH-HYW-0009 | hCG Pregnancy Rapid Test Midstream | Add To Cart |
| IH-HYW-0004 | Lh Ovulation Test Strip | Add To Cart |
| IH-HYW-0005 | Lh Ovulation Test Cassette | Add To Cart |
| IH-HYW-0006 | Lh Ovulation Test Midstream | Add To Cart |
| IH-HYW-0001 | hCG Pregnancy Test Strip | Add To Cart |
| IH-HYW-0008 | Pregnancy Rapid Combo Test Cassette (For Prescription Use) | Add To Cart |
| IH-HYW-0010 | hCG Early Pregnancy Rapid Test | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |