- Home
- Resource
- Explore & Learn
- Unveiling the Battle Against HPV: From Traditional Tests to Cutting-Edge Detection Technologies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Human papillomavirus (HPV) represents a formidable global health challenge, with over 100 distinct types identified. Among these, at least 14 high-risk strains are carcinogenic, with HPV-16 and HPV-18 accounting for approximately 70% of cervical cancer cases. The virus's ability to remain dormant in the body, often without any visible symptoms, allows precancerous lesions to develop insidiously. In 2020, the World Health Organization's 90-70-90 strategy set ambitious targets to combat cervical cancer. However, the realization of these goals hinges significantly on the effectiveness of HPV detection methods, particularly in low- and middle-income countries (LMICs) where the disease burden is disproportionately high.
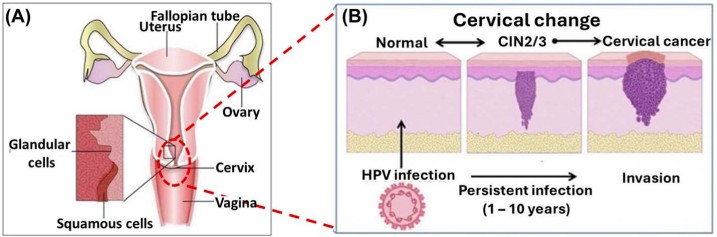 Fig.1 (A) Pictorial depiction of the cervix anatomy (B) schematic illustration of the cervical changes caused by HPV infection. (Fashedemi O. O., et al., 2025)
Fig.1 (A) Pictorial depiction of the cervix anatomy (B) schematic illustration of the cervical changes caused by HPV infection. (Fashedemi O. O., et al., 2025)
The Papanicolaou (Pap) smear, discovered in the 1940s, has been a cornerstone of cervical cancer screening. By examining cervical cells under a microscope, it has been instrumental in reducing cervical cancer mortality by up to 70% in regions with widespread adoption. Nevertheless, its limitations are substantial. The Pap smear suffers from relatively low sensitivity and specificity, frequently missing early HPV infections and yielding false-negative results. Moreover, its reliance on highly trained cytologists for interpretation poses a significant bottleneck, especially in areas with scarce medical resources. The variability in results among different observers also undermines its consistency in healthcare settings.
Nucleic acid-based techniques, such as real-time polymerase chain reaction (PCR), offer enhanced sensitivity and specificity by detecting HPV DNA. These methods can identify specific viral genotypes and quantify the viral load. However, they come with their own set of challenges. Requiring specialized laboratories and trained personnel, nucleic acid tests are often inaccessible in resource-poor regions. The time-consuming and costly nature of PCR tests further restricts their use for mass screening initiatives in LMICs. Additionally, the risk of contamination can lead to false-positive results, triggering unnecessary follow-up procedures and causing undue patient anxiety.
Electrochemical Biosensors: Miniaturized and Efficient
Electrochemical biosensors have emerged as a promising alternative for HPV detection. These devices utilize electrical signals to identify HPV biomarkers, offering several key advantages. They can deliver results in a matter of minutes, significantly faster than traditional methods. Their miniature and portable designs make them ideal for point-of-care (POC) testing, enabling access to screening in remote and underserved areas. Furthermore, electrochemical biosensors are cost-effective, requiring minimal reagents and being amenable to large-scale production. For instance, screen-printed electrodes (SPEs) combined with HPV-specific DNA probes have demonstrated the ability to detect HPV-16 DNA with a detection limit as low as 2 picomolar (pM).
CRISPR Technology: Gene-Editing Repurposed for Detection
The CRISPR-Cas9 system, well-known for its gene-editing capabilities, is now being repurposed for HPV detection. This technology offers unparalleled sensitivity, capable of detecting even minute amounts of HPV DNA at the attomolar level. Results can be obtained in as little as 20 minutes, providing a rapid diagnostic solution. The relative simplicity of the CRISPR-based detection method, with fewer sample preparation steps, makes it more accessible for widespread use. Researchers have successfully combined CRISPR with surface-enhanced Raman scattering (SERS) to create a highly sensitive system that can detect HPV-16 and HPV-18 at atomolar concentrations, opening new possibilities for POC testing.
Fourier Transform Infrared (FTIR) Spectroscopy: Molecular Fingerprinting
FTIR spectroscopy analyzes the unique molecular fingerprints of cells and tissues, making it a powerful tool for detecting HPV-induced changes. This non-invasive technique eliminates the need for tissue biopsies, reducing patient discomfort. It can simultaneously analyze multiple biomolecules, providing a comprehensive view of cellular alterations associated with HPV infection. FTIR offers real-time results, making it suitable for on-the-spot screening. Studies have shown that FTIR can effectively distinguish between HPV-positive and normal cervical cells by identifying characteristic spectral patterns. Specific peaks in the FTIR spectra of HPV-positive cells at certain wavenumbers correspond to changes in proteins and lipids, facilitating accurate diagnosis.
Raman Spectroscopy and SERS: High-Resolution Molecular Imaging
Raman spectroscopy and its enhanced variant, SERS, enable high-resolution molecular imaging, allowing for detailed visualization of the molecular changes caused by HPV infection. These techniques can detect subtle alterations in cellular components that precede visible morphological changes, facilitating early detection. SERS, in particular, can be integrated with other technologies, such as CRISPR or electrochemical sensors, to further enhance detection sensitivity. Nanosensor platforms based on SERS, using bimetallic gold-silver nanorods, have been developed to detect specific HPV genotypes with high accuracy, generating strong signals upon binding to HPV DNA.
Paper-Based Sensors: Inexpensive and User-Friendly
Paper-based analytical devices (PADs) are transforming POC testing for HPV. Made from inexpensive and widely available paper, these sensors are highly affordable. They are designed to be user-friendly, requiring minimal training for operation. Their lightweight and compact nature makes them easily transportable to remote locations. For example, a paper-based sensor that uses colorimetric changes in gold nanoparticles to detect HPV DNA has been developed. This sensor can be read using a smartphone, eliminating the need for specialized equipment and making HPV screening accessible to a broader population.
Smartphone-Based Platforms: Leveraging Digital Innovation
Smartphones are playing an increasingly important role in HPV screening. They can be used for image analysis, capturing, and examining cervix images to assist in visual inspection. Smartphones also enable data storage and secure transmission, allowing for remote consultation and diagnosis. User-friendly apps can guide individuals through the testing process and present results in an easily understandable format. In projects like the one in Kenya, smartphones were used to capture images after visual inspection with acetic acid (VIA). While initial sensitivity levels were a concern, this technology holds great promise for improving screening access and reducing diagnostic subjectivity.

Despite significant progress in HPV detection technologies, several challenges remain. Many innovative technologies have shown promise in laboratory settings but require extensive validation in diverse real-world clinical environments. Navigating complex regulatory frameworks is also a hurdle to bringing new diagnostic devices to market. Additionally, comprehensive training programs are needed to ensure healthcare workers are proficient in using new testing methods, and public awareness campaigns are essential to promote acceptance of these novel screening approaches.
However, the future is bright. Integrating multiple technologies, such as combining electrochemical sensors with artificial intelligence-powered data analysis, has the potential to create intelligent POC devices with unprecedented accuracy. Exploring non-invasive biomarkers, such as urinary proteins, for self-sampling methods could further increase screening participation rates. With continued research and development, we are edging closer to a future where HPV screening is universal, affordable, and highly effective, bringing us nearer to the goal of eliminating cervical cancer as a public health threat.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |