- Home
- Resource
- Disease Diagnosis
- Autoimmune Diseases
- Unraveling Pernicious Anemia: Essential Tests and Biomarkers for Accurate Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Pernicious anemia is an autoimmune disorder that leads to severe vitamin B12 deficiency through the destruction of gastric intrinsic factor. This resource provides a comprehensive guide to its modern diagnosis, detailing the essential laboratory pathway from initial suspicion—based on hematologic and neurologic symptoms—through the confirmation of deficiency via serum B12 and metabolic biomarkers, and finally to the establishment of its autoimmune etiology with specific serological tests.
Pernicious anemia is an autoimmune disorder characterized by the immune system attacking the parietal cells of the stomach, leading to the loss of intrinsic factor production and chronic inflammation of the gastric mucosa (atrophic gastritis). This results in the inability to absorb dietary vitamin B12, causing a severe and progressive deficiency. The condition manifests clinically with symptoms of megaloblastic anemia, such as fatigue and pallor, and can also cause significant neurological complications, including paresthesia, ataxia, and cognitive disturbances, due to the essential role of B12 in nerve function. Diagnosis is not based on symptoms alone but requires specific laboratory confirmation of B12 deficiency alongside serological evidence of autoimmune gastric involvement.
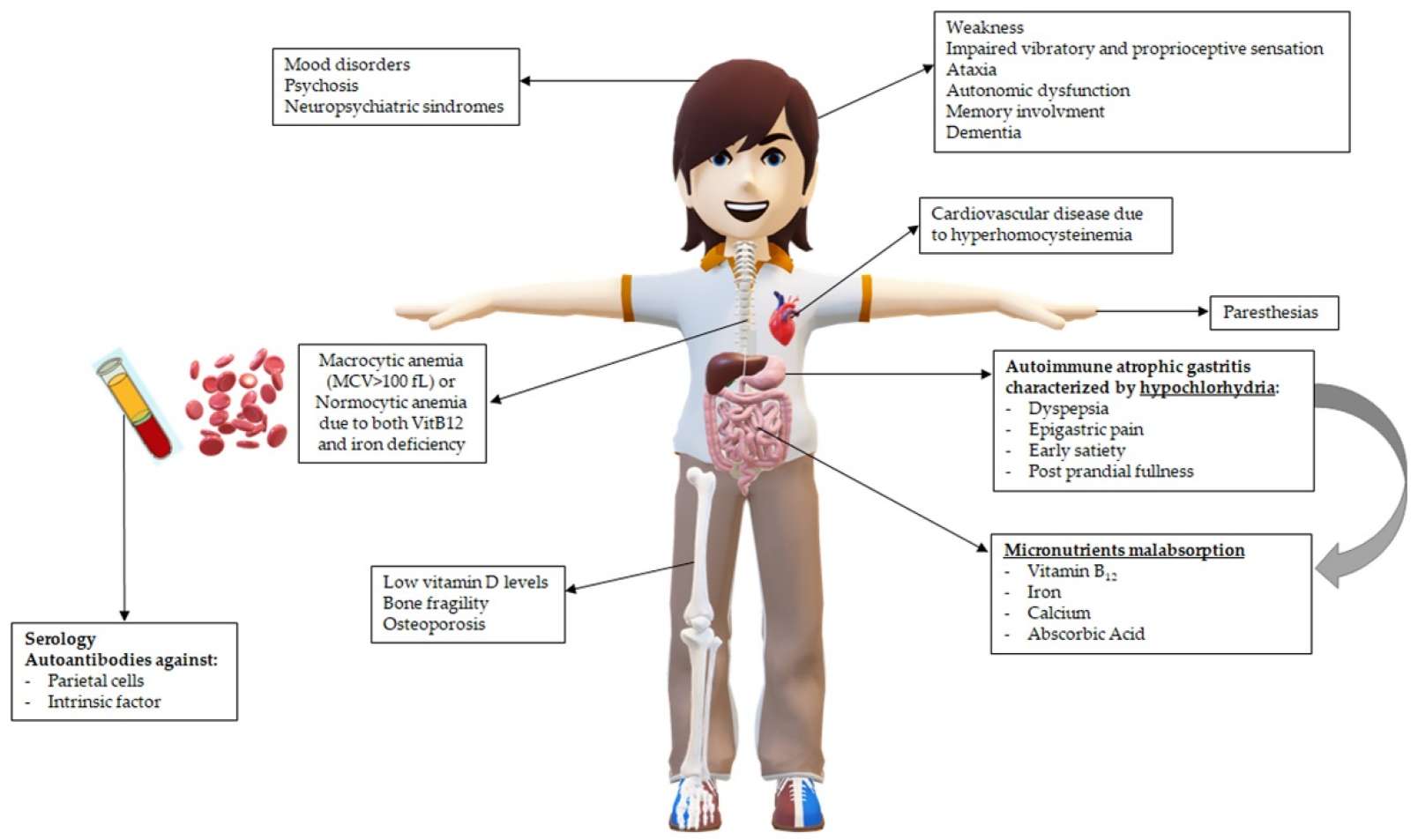 Fig.1 Main clinical consequences in patients with pernicious anemia (PA) related to hypochlorhydria and vitamin B12 deficiency. (Esposito, Gianluca, et al., 2022)
Fig.1 Main clinical consequences in patients with pernicious anemia (PA) related to hypochlorhydria and vitamin B12 deficiency. (Esposito, Gianluca, et al., 2022)
Clinical suspicion of pernicious anemia arises from recognizing a constellation of non-specific but suggestive signs, particularly in high-risk individuals. Key triggers include symptoms of megaloblastic anemia (severe fatigue, weakness, pallor, shortness of breath) often accompanied by distinctive neurological manifestations such as symmetric paresthesia (numbness/tingling in hands and feet), gait instability (ataxia), and cognitive changes like memory loss or confusion. Other clues include a smooth, red, painful tongue (glossitis), and a personal or family history of other autoimmune disorders (e.g., thyroid disease, type 1 diabetes, vitiligo). The purpose of clinical assessment is not to diagnose definitively at the bedside, but to identify this pattern—especially the combination of unexplained anemia with neurological or oral signs—which then mandates a specific and systematic laboratory investigation to confirm vitamin B12 deficiency and its autoimmune cause.
The first critical step in diagnosing pernicious anemia is to objectively confirm the presence of vitamin B12 deficiency at both the hematologic and cellular metabolic levels. This involves a triad of laboratory tests that move from screening blood cell abnormalities to directly measuring the vitamin and, most sensitively, assessing its functional metabolic consequences within the body.
Complete Blood Count (CBC) with Indices
The CBC serves as the initial screening tool. The classic hematologic hallmark of B12 deficiency is macrocytic anemia, identified by an elevated mean corpuscular volume (MCV), typically above 100 fL. Accompanying signs may include low hemoglobin and hematocrit, and an increased red cell distribution width (RDW) indicating a mix of cell sizes. It is crucial to note that neurological symptoms can precede these obvious blood changes, so a normal CBC does not rule out deficiency.
Serum Vitamin B12 Assay
This test directly measures the circulating concentration of vitamin B12 in the blood and is the primary initial confirmatory test. A level below the reference range (often <200 pg/mL) supports the diagnosis. However, interpretation requires caution due to significant limitations: assays have variable performance, and levels in the "low-normal" or borderline range (200-300 pg/mL) can still be associated with clinical deficiency, necessitating further metabolic testing.
Metabolic Biomarkers: MMA and Homocysteine
These functional biomarkers are essential for confirming true cellular deficiency, especially in equivocal cases. Serum methylmalonic acid (MMA) accumulates when B12 is deficient and is the most specific indicator. Total homocysteine rises in both B12 and folate deficiency. Elevated levels of one or both metabolites confirm a functional B12 deficit at the tissue level, offering high sensitivity and specificity even when serum B12 levels are inconclusive.
Once vitamin B12 deficiency is confirmed, the next diagnostic imperative is to determine if the underlying cause is the autoimmune process specific to pernicious anemia (PA). This involves serological testing for autoantibodies targeting key components of the stomach, which directly link the deficiency to its pathogenic origin and differentiate PA from other causes of B12 malabsorption.

The intrinsic factor antibody (IFA) test is the gold-standard serological marker for confirming pernicious anemia. It detects antibodies that directly block the binding site of intrinsic factor (IF) or form a complex with it, preventing B12 absorption. A positive result is highly specific (>95%) for PA and is considered diagnostic, even in the absence of other tests. It has moderate sensitivity (~50-70%), meaning a negative result does not completely rule out the disease, especially early on.
The parietal cell antibody (PCA) test detects antibodies against the gastric proton pump (H+/K+ ATPase) in parietal cells. It has high sensitivity (~80-90%) but low specificity, as it is also positive in other autoimmune gastritis and in some healthy individuals, particularly the elderly. Therefore, a positive PCA is a strong supportive finding, especially when IFA is negative, but it cannot establish a definitive diagnosis on its own.
These markers provide indirect evidence of the gastric atrophy characteristic of advanced PA. Elevated serum gastrin levels are common due to loss of acid-producing parietal cells and consequent loss of negative feedback. A low serum pepsinogen I level reflects the loss of chief cells. While not diagnostic alone, these markers support the diagnosis in a suggestive clinical and serological context and can help assess the extent of gastric damage.
Even with characteristic findings, diagnosing pernicious anemia (PA) can be challenging and requires careful navigation to exclude other conditions. Key complexities include patients with borderline serum B12 levels, negative autoantibodies (especially early in the disease), or confounding factors like concomitant folate deficiency or medication use (e.g., metformin, proton pump inhibitors). The differential diagnosis is broad and necessitates a systematic approach to rule out nutritional B12 deficiency (inadequate dietary intake, often in vegans/elderly), malabsorption syndromes (e.g., Crohn's disease, celiac disease, pancreatic insufficiency), drug-induced effects, and other causes of macrocytic anemia (e.g., myelodysplastic syndrome, hypothyroidism, alcohol overuse). In ambiguous cases, additional investigations such as Helicobacter pylori testing, endoscopy with gastric biopsy to directly assess for atrophic gastritis, or a therapeutic trial of B12 supplementation may be warranted to arrive at a definitive conclusion and ensure appropriate management.
By focusing on the critical biomarkers in the diagnostic algorithm, Alta DiagnoTech offers a comprehensive in vitro diagnostic (IVD) portfolio specifically designed to support the complete diagnostic pathway for pernicious anemia, from initial deficiency screening to definitive autoimmune confirmation. Our solutions encompass precise assays for these key biomarkers, leveraging advanced technologies to deliver the sensitivity and specificity required for accurate diagnosis and effective differential diagnosis in complex clinical scenarios. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Complete Blood Count (CBC) with Indices Analyzer | Automated Hematology Analyzer with Flow Cytometry and Impedance Technology | Initial Hematologic Screening: Provides critical data on red blood cell indices, including mean corpuscular volume (MCV) and red cell distribution width (RDW), to identify macrocytic anemia, the hallmark hematologic presentation. |
| Vitamin B12 (Cobalamin) Assay Kit | Chemiluminescent Microparticle Immunoassay (CMIA) | Confirming Deficiency: Precisely quantifies serum vitamin B12 levels to directly assess deficiency status, serving as the primary biochemical test in the diagnostic workup. |
| Methylmalonic Acid (MMA) & Homocysteine Assay Kits | Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) | Functional Metabolic Confirmation: Measures elevated levels of MMA and Homocysteine, the most sensitive and specific functional biomarkers of cellular B12 deficiency, crucial for diagnosis in borderline or equivocal B12 cases. |
| Intrinsic Factor Antibody (IFA) Assay Kit | Automated Chemiluminescent Immunoassay (CLIA) | Definitive Etiologic Diagnosis: Detects specific antibodies against intrinsic factor. A positive result is highly specific for confirming the autoimmune etiology of pernicious anemia. |
| Parietal Cell Antibody (PCA) Assay Kit | Automated Chemiluminescent Immunoassay (CLIA) | Supportive Serological Marker: Detects antibodies against gastric parietal cells. A positive result supports the diagnosis of autoimmune gastritis, especially when IFA is negative. |
| Automated Immunoassay Analyzer System | Modular, High-Throughput Immunoassay Platform | Integrated Diagnostic Workflow: A scalable and reliable instrument system designed to run the full serological and metabolic panel (B12, IFA, PCA, etc.) with high efficiency and standardization, streamlining the diagnostic process. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |