- Home
- Resource
- Explore & Learn
- Unlocking the Mysteries of Serological Diagnostics: A Comprehensive Guide to Validation and Quality Assurance
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Serological diagnostics have become indispensable tools in modern healthcare, enabling the detection of antibodies and antigens in biological samples. These tests are pivotal in diagnosing infectious diseases, autoimmune disorders, and even chronic conditions like cancer. The accuracy and reliability of serological diagnostics hinge on rigorous validation and quality assurance processes. This comprehensive guide delves into the intricacies of validating serological assays, ensuring their performance meets the highest standards of precision and reliability.
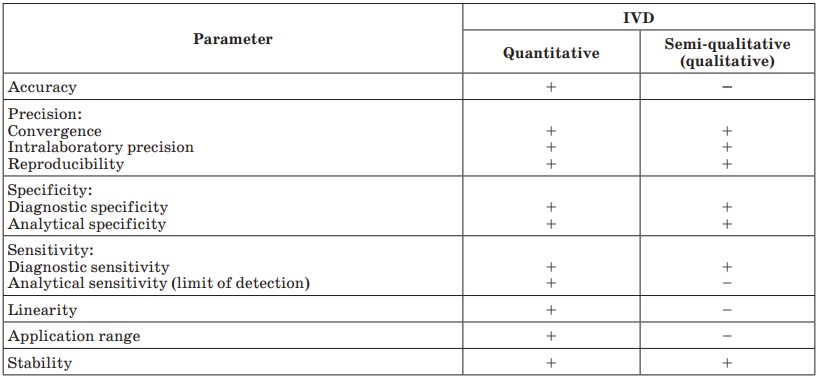 Fig.1 Validation parameters for different kinds of IVD. (Galkin O. Y., et al., 2018)
Fig.1 Validation parameters for different kinds of IVD. (Galkin O. Y., et al., 2018)
Serological assays rely on the interaction between antibodies and antigens. When a pathogen invades the body, the immune system produces specific antibodies to neutralize it. Serological tests detect these antibodies or the antigens themselves in blood, serum, or other bodily fluids. The most common types of serological assays include enzyme-linked immunosorbent assays (ELISAs), lateral flow assays (LFAs), and chemiluminescent immunoassays (CLIAs).
Table 1: Types of Controls in Serological Assays.
| Control Type | Purpose | Example |
| Positive Control | Validates assay sensitivity | Known antibody-positive serum |
| Negative Control | Validates assay specificity | Antibody-negative serum |
| Internal Control | Monitors assay consistency | Housekeeping protein in ELISA |

Sensitivity and Specificity
Sensitivity measures the assay's ability to correctly identify true positives, while specificity measures its ability to correctly identify true negatives. High sensitivity is crucial for detecting low-titer antibodies in early-stage infections, whereas high specificity minimizes false positives, which can lead to unnecessary treatments or quarantines.
Example: In a study evaluating an ELISA for detecting antibodies against SARS-CoV-2, the assay demonstrated 98% sensitivity and 99% specificity, making it highly reliable for diagnostic purposes.
Precision and Reproducibility
Precision refers to the assay's ability to produce consistent results under the same conditions. Reproducibility assesses whether the assay yields similar results across different laboratories or operators. Both are critical for ensuring that serological tests provide reliable data in clinical settings.
Method: To evaluate precision, run the same sample multiple times (intra-assay precision) and across different days or operators (inter-assay precision). Calculate the coefficient of variation (CV) to quantify variability.

Limit of Detection (LoD) and Limit of Quantitation (LoQ)
The LoD is the lowest concentration of an analyte that can be reliably detected, while the LoQ is the lowest concentration that can be quantitatively determined with acceptable precision and accuracy. Determining these limits ensures that the assay can detect clinically relevant levels of antibodies or antigens.
Example: For an HIV antibody test, the LoD might be set at 0.1 IU/mL, ensuring that even low levels of antibodies are detected, which is critical for early diagnosis.
GMP compliance is mandatory for manufacturing serological diagnostic kits. GMP ensures that products are consistently produced and controlled according to quality standards. Key aspects include:
QC materials are used to monitor the performance of serological assays during production and after release. These include:
Table 2: Types of QC Materials in Serological Diagnostics.
| QC Material Type | Purpose | Frequency of Use |
| Positive QC | Validates sensitivity | Daily |
| Negative QC | Validates specificity | Daily |
| Stability QC | Monitors reagent stability | Monthly |
| Precision QC | Assesses reproducibility | Quarterly |
Serological diagnostic kits must comply with international and national regulations, such as the FDA (U.S.), CE (Europe), and local health authorities. Compliance involves:
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| AADA-HMM-0004 | Dextromethorphan (DXM)-02 | Add To Cart |
| AAKIM-HMM-0002 | Urinary Microalbumin (HSA[MAU])-04 | Add To Cart |
| AADA-HMM-0006 | Dextromethorphan (DXM)-40 | Add To Cart |
| AACB-HMM-0012 | Procalcitonin (PCT)-01 | Add To Cart |
| AACB-HMM-0004 | Heart-type Fatty Acid-bindin Protein (H-FABP)-01 | Add To Cart |
| AAID-HMM-0007 | Influenza A Virus (FluA)-09 | Add To Cart |
| AACB-HMM-0015 | Procalcitonin (PCT)-09 | Add To Cart |
| AALIM-HMM-0003 | Glycocholic Acid (CG) -98 | Add To Cart |
| AADA-HMM-0003 | Etomidate (ETO) | Add To Cart |
| AACB-HMM-0009 | D-Dimer-06 | Add To Cart |
| AACB-HMM-0010 | D-Dimer-10 | Add To Cart |
| AACB-HMM-0002 | N-terminal pro-brain natriuretic peptide (NT-ProBNP)-02 | Add To Cart |
| AATM-HMM-0005 | Carbohydrate Antigen 125 (CA125)-01 | Add To Cart |
| CLRM-HMM-0002 | DNP-BSA | Add To Cart |
| AATM-HMM-0003 | Ferritin-03 | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |