- Home
- Resource
- Explore & Learn
- Unlocking Diagnostic Innovation: How a Mini-Bioreactor is Revolutionizing PCR Testing
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Infectious disease diagnostics is a critical component of public health. The ability to quickly and accurately diagnose diseases directly impacts patient care, treatment decisions, and the containment of outbreaks. Polymerase Chain Reaction (PCR) has emerged as a cornerstone in diagnostics, particularly for detecting pathogens at the molecular level. However, the PCR process relies heavily on the availability of specific reagents, particularly deoxynucleoside triphosphates (dNTPs), which are essential for DNA replication during the test.
In many parts of the world, especially in low-resource settings, access to these essential diagnostic reagents remains a significant barrier. The COVID-19 pandemic underscored this issue, revealing vulnerabilities in the global supply chain and highlighting the need for local, on-demand production of key reagents. A novel approach to address this problem is the development of a mini-bioreactor capable of producing dNTPs on-site. This mini-bioreactor system offers a cost-effective, sustainable solution that could transform PCR testing, particularly in resource-limited environments.
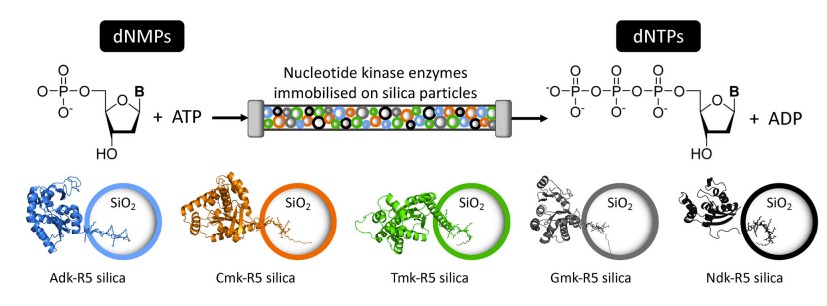 Fig.1 Schematic of bioreactor device containing silica particles coated with kinase-R5 fusion proteins. (Bird A. R., et al., 2024)
Fig.1 Schematic of bioreactor device containing silica particles coated with kinase-R5 fusion proteins. (Bird A. R., et al., 2024)
One of the primary obstacles in expanding access to diagnostic tests is the availability and cost of reagents. In many developing countries, the lack of local production of diagnostic components, such as dNTPs, creates dependency on international supply chains. These supply chains are often unreliable and costly, making it difficult for low- and middle-income countries (LMICs) to access the necessary components for PCR testing.
During the COVID-19 pandemic, global supply chains for PCR reagents were severely disrupted. Many LMICs faced significant delays in obtaining these critical supplies, hindering their ability to respond to the pandemic effectively. The situation highlighted the need for self-sufficiency in diagnostic reagent production, particularly for vital ingredients like dNTPs, which are a prerequisite for most PCR tests.
Deoxynucleoside triphosphates (dNTPs) are the building blocks required for the amplification of DNA in PCR. Each dNTP consists of a deoxynucleoside (adenosine, cytosine, guanine, or thymine) linked to three phosphate groups. During PCR, DNA polymerase uses these dNTPs to synthesize new DNA strands. The four types of dNTPs—dATP, dCTP, dGTP, and dTTP—are essential for accurate DNA replication and amplification.
Traditionally, these dNTPs are purchased from commercial suppliers, often in pre-mixed formats to simplify PCR procedures. However, this reliance on external sources can be problematic, particularly in regions where supply chains are weak or expensive. The cost of commercial dNTPs can also be prohibitive, making PCR testing inaccessible for some populations. The challenge, then, is how to produce these essential reagents locally, efficiently, and cost-effectively.
The mini-bioreactor developed by researchers at the University of Cambridge represents a significant breakthrough in the field of diagnostics. This device utilizes a novel enzymatic process to produce dNTPs on-site using immobilized enzymes, offering a self-contained, reusable solution for PCR reagent production. The bioreactor system is designed to produce dNTPs from simpler nucleoside monophosphate precursors (dNMPs), which are then converted into the required dNTPs through enzymatic phosphorylation.
The mini-bioreactor relies on a set of immobilized kinases that are responsible for the conversion of dNMPs to dNTPs. These enzymes are immobilized on silica beads, which offer several advantages. First, silica is a highly stable material that ensures the enzymes remain active and functional over extended periods. Second, immobilization on silica prevents enzyme loss during the reaction process, allowing for multiple uses of the bioreactor.
The kinases used in the system are designed to be highly specific for their respective substrates, with each kinase targeting one of the four dNMPs (dAMP, dCMP, dGMP, and dTMP). The enzymes catalyze the transfer of phosphate groups from ATP to the dNMPs, producing the corresponding dNTPs required for PCR.
One of the key features of the mini-bioreactor is its reusability. The immobilized enzymes remain stable and active for up to two months when stored at 4°C. This stability allows the bioreactor to be used repeatedly without the need for constant replenishment of enzymes. In fact, studies have shown that the system can be reused for up to 12 cycles over the course of two months, making it a highly sustainable solution for on-demand dNTP production.
Additionally, the enzymes in the bioreactor have been shown to perform effectively even after extended storage. For example, after being stored at 4°C, the bioreactor was able to continue producing dNTPs that performed well in PCR tests, demonstrating that the system could function in low-resource settings where refrigeration and other conditions may not be ideal.
The true potential of this mini-bioreactor lies in its ability to produce high-quality dNTPs for real-world applications. The bioreactor has been tested in a variety of PCR experiments, including those targeting lambda DNA and Plasmodium malariae, the parasite responsible for malaria.
Lambda Genome PCR
In PCR tests targeting the lambda genome, the bioreactor-produced dNTPs successfully amplified DNA fragments up to 7.5 kb in length. This result is consistent with the expected performance for dNTPs in standard PCR procedures. The bioreactor’s ability to produce dNTPs for such large DNA fragments demonstrates its potential for use in a wide range of diagnostic applications.
Plasmodium malariae Detection
The performance of the bioreactor was also evaluated in detecting Plasmodium malariae through quantitative PCR (qPCR). The dNTPs produced by the mini-bioreactor showed a high correlation between the cycle threshold (Ct) values and the log of the copy number of the target DNA. This indicates that the bioreactor-produced dNTPs are suitable for sensitive and accurate pathogen detection, even at low copy numbers.
Moreover, the bioreactor-produced dNTPs showed fewer false positives compared to commercial dNTPs. This is a critical factor in ensuring the reliability of PCR tests, particularly in field diagnostics where sample contamination or reagent impurities can lead to erroneous results.
One of the most significant benefits of the mini-bioreactor is its potential for use in low-resource settings. Many regions, particularly in sub-Saharan Africa and Southeast Asia, lack the infrastructure to support large-scale diagnostic reagent production. This limitation often leads to delays in testing, misdiagnoses, and ineffective treatment.
The mini-bioreactor offers a portable, self-sufficient solution that can be deployed directly in field settings. It eliminates the need for external reagent supplies, allowing healthcare workers to produce dNTPs locally as needed. This reduces the reliance on expensive and often unreliable supply chains and provides a cost-effective alternative to traditional diagnostic reagents.
Furthermore, the mini-bioreactor's ability to operate without a constant supply of fresh reagents means that it can function in remote areas with limited access to commercial diagnostic products. This makes it an invaluable tool for health workers conducting on-site diagnostics in rural or isolated communities.
While the mini-bioreactor has shown great promise, further development is needed to optimize its performance and scalability. Currently, the system is designed for small-scale use, with the capacity to produce dNTPs for a limited number of PCR reactions. Scaling up the system for larger populations or higher throughput applications will require modifications to the bioreactor design, as well as improvements in enzyme efficiency and stability.
In addition, further research is needed to refine the enzyme immobilization process to increase the overall yield of dNTPs and reduce costs. Advances in bioreactor design and enzyme engineering could help make this technology more accessible and affordable for widespread use in diagnostic labs worldwide.
The mini-bioreactor represents a groundbreaking advancement in diagnostic reagent production, offering a sustainable, cost-effective solution for on-demand dNTP synthesis. Its potential to improve access to PCR testing in low-resource settings cannot be overstated. By enabling local production of essential reagents, the bioreactor empowers healthcare providers to conduct accurate and timely diagnoses, even in remote or underserved areas.
As research continues and the technology is optimized, the mini-bioreactor could become a key tool in global diagnostic infrastructure, helping to ensure that all individuals, regardless of their location or economic status, have access to the critical diagnostics needed to manage and treat infectious diseases.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |