- Home
- Resource
- Explore & Learn
- Transforming Hormone Monitoring: The Emergence Of Paper-Based Analytical Platforms
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Hormones are crucial biochemical messengers that regulate a myriad of physiological processes, including metabolism, growth, reproduction, and mood. Accurate detection of hormones is essential for diagnosing endocrine disorders, monitoring treatment efficacy, and ensuring overall health.
Traditional hormone detection methods, such as bioassays, immunoassays, and receptor assays, have been the cornerstone of diagnostic practices for decades. However, these methods come with several challenges. Bioassays, while providing physiological relevance, often lack specificity and sensitivity. Immunoassays, though highly sensitive, are prone to cross-reactivity and require expensive reagents and specialized equipment. Receptor assays, while offering high specificity, are limited by the availability of suitable receptors and the complexity of the assay setup.
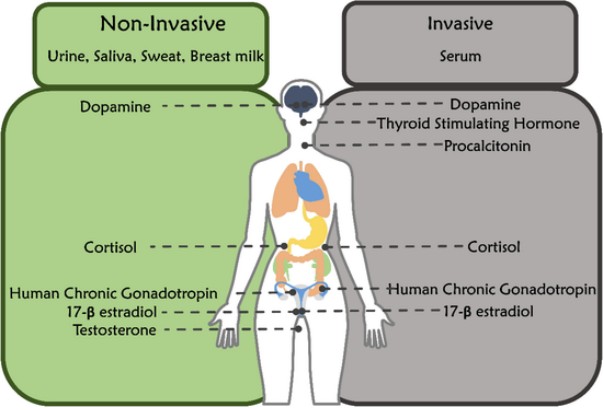 Fig.1 Different hormones in non-invasive and invasive body fluids. (Kelkar N., et al., 2022)
Fig.1 Different hormones in non-invasive and invasive body fluids. (Kelkar N., et al., 2022)
In response to the limitations of traditional methods, which often require expensive equipment, skilled personnel, and complex procedures, paper-based analytical devices have emerged as a truly revolutionary alternative. These innovative devices capitalize on the unique and inherent advantages of paper—its low cost, widespread availability, ease of fabrication, and seamless compatibility with a variety of printing methods—to create diagnostic tools that are not only simple and portable but also remarkably user-friendly. They have the potential to democratize diagnostic testing by making it accessible to a broader range of users, including those in resource-limited settings.
Paper-based devices operate on the sophisticated principles of microfluidics, where fluid samples are precisely manipulated within micro-scale channels and zones that are meticulously defined by hydrophobic barriers on the paper surface. This ingenious design allows for the seamless integration of multiple assay steps onto a single, compact platform. As a result, it facilitates rapid and highly efficient detection, significantly reducing the time and effort required for diagnostic processes. This advancement holds great promise for improving healthcare outcomes by enabling faster and more convenient testing options.


Colorimetric assays are among the most widely used methods in paper-based devices. These assays rely on the visible color change induced by a biochemical reaction, which can be easily observed with the naked eye or quantified using a smartphone camera and image analysis software.
For instance, a study by Zangheri et al. demonstrated the detection of cortisol in saliva using a chemiluminescent lateral flow immunoassay (LFIA) integrated into a smartphone. The assay employed a competitive immunoassay format, where cortisol-peroxidase conjugates reacted with a chemiluminescent substrate to produce a detectable signal. This method achieved rapid results (<30 minutes) and a dynamic detection range of 0.3 to 60 ng/mL.
Electrochemical sensors offer high sensitivity and selectivity for hormone detection. These sensors typically consist of electrodes modified with specific recognition elements, such as enzymes, antibodies, or aptamers, that interact with the target hormone to produce an electrical signal.
A notable example is the development of a paper-based electrochemical biosensor for cortisol detection in human saliva by Khan et al. This sensor utilized a graphene nanoplatelet and amphiphilic diblock copolymer composite coated on filter paper, with micro-Au electrodes deposited on top. The sensor exhibited a wide detection range from 1 pg/mL to 10 ng/mL and a response time of 10 minutes.

Fluorescence-based assays offer high sensitivity and the potential for multiplexed detection. These assays employ fluorescent dyes or quantum dots that emit light upon excitation, allowing for the quantification of target hormones.
For example, a study by Dalirirad and Steckl developed an aptamer-based lateral flow assay for cortisol detection in sweat using gold nanoparticles functionalized with cortisol-specific aptamers. The presence of cortisol caused the aptamers to desorb from the gold nanoparticles, which were then captured on a test zone, producing a visible fluorescent signal. This method achieved a dynamic range of 8 to 140 ng/mL and an analysis time of approximately 150 minutes.
Despite the growing interest in non-invasive body fluids, serum remains the gold standard for hormone detection due to its high biomarker concentration and stability. The robustness and reliability of serum as a diagnostic medium make it indispensable for accurate and comprehensive hormone analysis. Paper-based devices have been developed to detect a wide range of hormones in serum, including cortisol, dopamine, thyroid-stimulating hormone (TSH), and 17β-estradiol. These devices offer a cost-effective and portable alternative to traditional laboratory-based methods, making hormone testing more accessible and convenient.
For example, Apilux and colleagues fabricated a paper-based immunosensor with a competitive assay format for cortisol detection in serum. This innovative device employed gold nanoparticles conjugated with anti-cortisol antibodies as signal indicators, achieving a detection limit of 21.5 µg/dL and a rapid testing time of just 30 minutes. This advancement not only demonstrates the high sensitivity and specificity of paper-based devices but also highlights their potential for real-time, point-of-care diagnostics in clinical settings.
Beyond serum, paper-based devices have also been explored for hormone detection in other invasive body fluids, such as cerebrospinal fluid (CSF) and ascitic fluid. While these fluids are less accessible and often require more invasive procedures to obtain, they contain unique biomarkers that can provide invaluable diagnostic information for conditions such as neurological disorders and certain cancers.
For instance, research has shown that paper-based devices can be adapted to detect specific hormones and biomarkers in cerebrospinal fluid, offering a less cumbersome alternative to traditional CSF analysis methods. Similarly, the detection of hormones in ascitic fluid can aid in the diagnosis and monitoring of conditions like ovarian cancer. These applications underscore the versatility and adaptability of paper-based devices in addressing complex diagnostic needs across various body fluids.
Overall, the development of paper-based devices for invasive body fluid analysis represents a significant step forward in making advanced diagnostic tools more accessible, portable, and user-friendly, while maintaining the high standards of accuracy and reliability required for clinical diagnostics.
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| IH-HYW-0002 | hCG Pregnancy Test Cassette | Add To Cart |
| IH-HYW-0003 | hCG Pregnancy Test Midstream | Add To Cart |
| IH-HYW-0007 | ShinetelI™ Digital Pregnancy Test | Add To Cart |
| IH-HYW-0009 | hCG Pregnancy Rapid Test Midstream | Add To Cart |
| IH-HYW-0004 | Lh Ovulation Test Strip | Add To Cart |
| IH-HYW-0005 | Lh Ovulation Test Cassette | Add To Cart |
| IH-HYW-0006 | Lh Ovulation Test Midstream | Add To Cart |
| IH-HYW-0001 | hCG Pregnancy Test Strip | Add To Cart |
| IH-HYW-0008 | Pregnancy Rapid Combo Test Cassette (For Prescription Use) | Add To Cart |
| IH-HYW-0010 | hCG Early Pregnancy Rapid Test | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |