- Home
- Resource
- Explore & Learn
- The Vital Role of Rapid Diagnostic Tests in Global Pandemic Preparedness
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In the face of ongoing global health threats, the emergence of new infectious diseases and the re-emergence of others have necessitated the development of effective diagnostic tools to support pandemic preparedness. Rapid Diagnostic Tests (RDTs) are central to this effort, offering a swift and reliable means of detecting infectious agents at the point of care (POC). These tools enable health systems to identify and manage outbreaks quickly, minimizing the societal impact of pandemics. However, despite their critical role, RDTs face substantial barriers to development and widespread implementation. This article explores the significance of RDTs in global pandemic preparedness, the challenges to their deployment, and the path forward to enhance their capabilities in combating future global health emergencies.
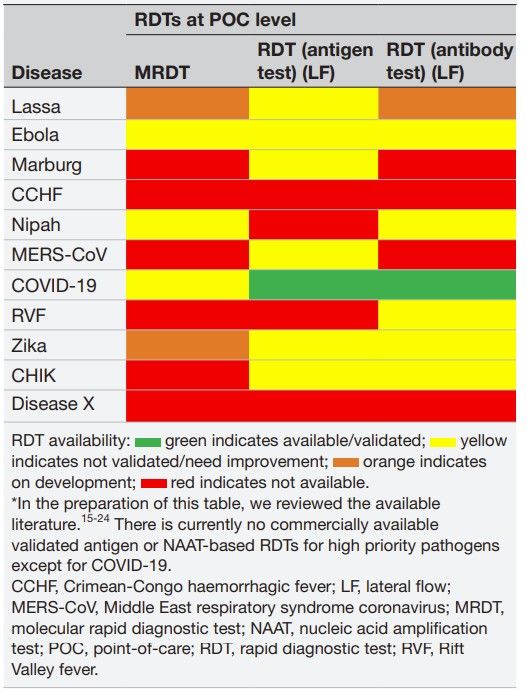 Fig.1 Inhouse and commercially available rapid diagnostic tests for high-priority pathogens. (Yimer S. A., et al., 2024)
Fig.1 Inhouse and commercially available rapid diagnostic tests for high-priority pathogens. (Yimer S. A., et al., 2024)

One of the most significant advantages of RDTs is their ability to deliver early detection of infectious diseases. Early diagnosis is pivotal in controlling the spread of contagious pathogens, especially in resource-limited settings. Infectious diseases often present with nonspecific symptoms, which can lead to delayed diagnoses if advanced laboratory testing is required. In remote or low-income regions, where access to specialized healthcare services is often limited, the availability of RDTs provides an essential solution for timely diagnosis. These tests can be performed in a variety of settings, including community clinics, homes, and even at mobile health units, without the need for sophisticated laboratory infrastructure.
Take, for example, the Ebola Virus Disease (EVD) outbreak in West Africa. The limited access to diagnostic tools contributed to delayed responses and worsened the spread of the virus. RDTs, particularly those based on lateral flow technology, could have significantly reduced diagnosis time, providing a quicker pathway for isolating infected individuals and preventing further spread. The ability to identify cases early is critical not only for patient management but also for epidemic containment and contact tracing efforts, which are foundational to preventing large-scale transmission.

Case Study: COVID-19 and Early Intervention
The COVID-19 pandemic served as a stark reminder of the importance of rapid testing in managing outbreaks. During the early stages of the pandemic, countries that implemented widespread testing saw better outcomes in containment and quicker responses to emerging hotspots. South Korea, for instance, achieved relatively early success in controlling the virus through large-scale diagnostic testing, contact tracing, and quarantine measures. In contrast, regions that faced testing delays, particularly those with limited diagnostic infrastructure, struggled to control the virus's spread.
RDTs, particularly antigen tests, became essential in the global response to COVID-19 by facilitating mass testing at the POC. These tests provided quick results, enabling individuals to self-isolate or seek treatment promptly. Such rapid interventions would have been nearly impossible without the availability of reliable RDTs.
In the wake of past pandemics, the global community has recognized the need for an accelerated response to emerging infectious diseases. The 100 Days Mission, a global initiative supported by organizations such as the Coalition for Epidemic Preparedness Innovations (CEPI), aims to have diagnostics, vaccines, and therapeutics ready for global deployment within 100 days of recognizing a new pathogen. For this mission to be successful, RDTs must be capable of providing accurate results within hours of a potential outbreak being identified.
The 100 Days Mission not only focuses on developing diagnostics for known pathogens like Nipah and Ebola, but also emphasizes preparedness for Disease X, a hypothetical unknown pathogen that could cause a future pandemic. To combat such a scenario, rapid and effective diagnostic tests are required to detect Disease X early, enabling prompt public health interventions such as isolation measures and contact tracing before vaccines and treatments are available. RDTs, with their ability to deliver real-time results without the need for complex laboratory systems, are key to enabling this rapid containment.

Despite their importance, the development of RDTs for high-priority pathogens remains significantly underfunded. Global diagnostics funding has historically been limited, with the majority of financial resources directed towards vaccine and therapeutic development. This disparity is particularly pronounced in the context of emerging infectious diseases, where diagnostic tools often do not receive adequate investment. The lack of market incentives also plays a role in hindering the development of RDTs. Since many high-priority diseases, such as Lassa fever and Nipah virus, cause localized outbreaks, the market for diagnostic tests is often too small to justify investment by private companies.
The result is a lag in the availability of accurate and validated RDTs for these diseases. As of now, there are no validated antigen-based or nucleic acid amplification tests (NAATs) for high-priority pathogens like MERS-CoV or Marburg virus. This gap underscores the need for substantial investment from both public and private sectors to support RDT development and ensure they are ready for deployment in the event of an outbreak.

Developing and validating RDTs also involves overcoming significant regulatory hurdles. The process of clinical validation, which ensures that an RDT is both sensitive and specific, is often slow and costly. This is exacerbated by a lack of well-characterized patient samples and inadequate research infrastructure, particularly in low-income and middle-income countries (LMICs). Many endemic regions that bear the brunt of high-priority infectious diseases also lack the necessary infrastructure for diagnostic research, making it difficult to validate new diagnostic assays.
Furthermore, regulatory pathways for approving RDTs can vary widely between regions, further complicating the global harmonization of testing standards. For example, in Africa, where diseases like Ebola and Marburg pose a significant threat, regulatory frameworks are often not aligned across countries, delaying the introduction of effective diagnostics.
Strengthening Local Manufacturing Capabilities
To ensure that RDTs are available when needed, there must be a concerted effort to strengthen local manufacturing capabilities in regions most vulnerable to outbreaks. Local production of diagnostic tools reduces reliance on global supply chains and ensures that diagnostic tests can be rapidly deployed in response to regional health threats. Manufacturing capacity in Africa, for example, is currently insufficient to meet the demand for diagnostic tools during a health crisis, leading to delays in deployment and contributing to the underdiagnosis of infectious diseases.
Increased investment in local manufacturing facilities, along with technology transfer programs and skills development initiatives, will enable countries to respond more effectively to emerging diseases. Partnerships between governments, international organizations, and private industry are crucial for scaling up these manufacturing capacities and ensuring that diagnostic tools are available locally during outbreaks.
Regulatory Harmonization and International Collaboration
International cooperation is another key component in improving the global diagnostic landscape. Organizations like the World Health Organization (WHO) and PATH have already undertaken significant efforts to create global frameworks for diagnostic development and deployment. For example, the REASSURED criteria developed by WHO outlines the key features that RDTs must possess to be effective in low-resource settings: real-time connectivity, ease of specimen collection, affordability, sensitivity, specificity, user-friendliness, rapid turnaround, robustness, and environmental sustainability. These criteria should guide the development and deployment of RDTs across various settings.
Furthermore, initiatives like the Africa CDC's Diagnostic Advisory Committee (DAC) are working to harmonize regulatory processes and increase collaboration between African countries. By aligning regulatory standards and improving local research capacity, these initiatives aim to make diagnostic tools more accessible and effective across the continent.
The COVID-19 pandemic underscored the significant disparities in access to diagnostic tools. High-income countries were able to quickly ramp up testing capacity, while many low-income countries (LICs) faced severe shortages in diagnostic supplies. The inequity in access to diagnostics led to delays in identifying cases, delayed containment measures, and escalating public health risks in these regions. Moving forward, ensuring equitable access to diagnostics must be a priority in pandemic preparedness.
Global initiatives such as COVAX, which aimed to ensure equitable access to COVID-19 vaccines, can serve as a model for ensuring access to diagnostic tests in future pandemics. Pooled procurement, subsidized pricing, and donor-funded programs can help address the issue of diagnostic access inequality.
Investment in global health infrastructure, particularly in LICs, is necessary to ensure that diagnostic tools are not only available but also accessible. This includes investing in laboratory facilities, biobanks for sample storage, and research networks for the continuous development of new diagnostic technologies. Governments and international organizations must increase their support for building these infrastructures to ensure that rapid diagnostics can be deployed when an outbreak occurs.
RDTs play a pivotal role in the fight against infectious disease outbreaks, providing early detection, enabling prompt case management, and supporting effective epidemic control measures. However, to maximize their impact, significant efforts must be made to overcome the barriers hindering their development, validation, and global deployment. This includes increasing investment in research, strengthening regulatory pathways, and ensuring that RDTs are accessible to all regions, particularly those most at risk of future pandemics.
By improving the capabilities and accessibility of RDTs, the global community will be better equipped to respond to the challenges of emerging infectious diseases and prevent future pandemics from spiraling out of control. The urgency of the situation requires immediate action from governments, international organizations, and the private sector to prioritize RDTs as a cornerstone of global pandemic preparedness.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |