- Home
- Resource
- Explore & Learn
- The Remarkable Journey: How In Vitro Diagnostics Transformed Healthcare
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Clinical chemistry stands as the bedrock of in vitro diagnostics, analyzing body fluids to assess organ function and metabolic status. In the early days, tests were rudimentary. For instance, glucose measurement relied on the o-Toluidine method, which involved complex chemical reactions with limited specificity. Cholesterol assessment used the Liebermann-Burchard method, requiring painstaking manual procedures and offering only rough estimations. These methods were time-consuming, prone to human error, and difficult to automate, severely limiting their utility in high-volume laboratories.
The advent of enzymatic methods in the 1990s was a game-changer. Take glucose oxidase, which replaced the o-Toluidine method. This enzyme specifically targets glucose, triggering a biochemical reaction that provides a more accurate and reliable measurement. Cholesterol oxidase followed suit for cholesterol testing. These enzymatic assays required smaller sample volumes, reduced reagent consumption, and could be integrated into automated analyzers. Today, most clinical chemistry tests utilize single, liquid-stable, ready-to-use reagents. For example, in creatinine measurement, while still relying on some chemical principles, the process has been refined to be more efficient and precise. The introduction of thin-film dry slide technology in "closed systems" further streamlined the testing process, allowing for rapid and consistent results across a wide range of analytes.
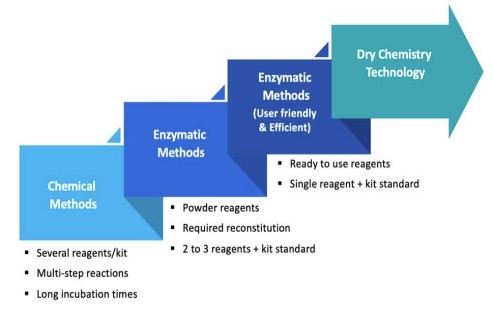 Fig.1 Evolution of Testing Methods in Clinical Chemistry. (Coro F., et al., 2025)
Fig.1 Evolution of Testing Methods in Clinical Chemistry. (Coro F., et al., 2025)
Immunology tests play a pivotal role in detecting antibodies and antigens, crucial for diagnosing infections, autoimmune diseases, and hormonal imbalances. In the 1980s, latex-based agglutination tests were prevalent. For pregnancy detection, these tests use latex particles coated with antibodies against human chorionic gonadotropin (hCG). When hCG was present in the urine sample, the latex particles would agglutinate, indicating a positive result. However, these tests suffered from low sensitivity and specificity, often leading to false positives or negatives.
The 1990s witnessed the rise of immunochromatography and colloidal gold-based tests. Home pregnancy tests, a prime example, became widely available and user-friendly. These tests use a strip with immobilized antibodies that react with the target antigen (hCG), producing a visible line for a positive result. This innovation brought point-of-care testing to the forefront, enabling rapid results without the need for a laboratory setting.
The transition from Radioimmunoassay (RIA) to safer alternatives was another significant milestone. RIA, used for measuring hormones like thyroid-stimulating hormone (TSH) and insulin, employed radioactive reagents. While it offered good sensitivity, the associated radiation hazards, strict regulatory requirements for handling radioactive materials, and the need for specialized training limited its use. Enzyme-Linked Immunosorbent Assay (ELISA) emerged as a superior alternative. By replacing radioactive reagents with enzymes, ELISA could detect antigen-antibody reactions with high sensitivity and specificity. For instance, the ELISA test for HIV screening in blood banks has a sensitivity of 99.5% and specificity of 98%. ELISA has evolved through four generations, with each generation improving detection speed and accuracy. The fourth-generation test, for example, can detect both HIV antibodies and the p24 antigen, significantly reducing the window period for diagnosis.
Chemiluminescence Immunoassays (CLIA) further enhanced immunology testing. CLIA uses chemical reactions that produce light, detected by a luminometer. With 10 times the sensitivity of ELISA and full automation capabilities, CLIA is now the preferred method for quantitative hormone and vitamin assays, providing a wide dynamic range and highly reliable results.
Hematology, centered around the Complete Blood Count (CBC), provides essential insights into blood cell health. In the past, CBCs were performed manually. Technicians would prepare blood smears, stain them, and painstakingly count red blood cells, white blood cells (WBCs), and platelets under a microscope. This method was labor-intensive, time-consuming, and subject to inter-operator variability.
The invention of the Coulter counter in the 1950s revolutionized hematology. Based on the principle of impedance, it measured the electrical resistance change as cells passed through a small aperture, allowing for automated cell counting. This technology paved the way for the development of more advanced hematology analyzers.
The evolution of CBC analyzers can be seen in their increasing ability to classify WBC subpopulations. 2-part differential analyzers could only distinguish between granulocytes and lymphocytes/monocytes. 3-part differential analyzers added the ability to identify monocytes separately. 5-part differential analyzers took a giant leap by precisely counting neutrophils, lymphocytes, basophils, eosinophils, and monocytes. This detailed classification is crucial for diagnosing infections, as different types of WBCs respond differently to various pathogens. For example, an increase in eosinophils may indicate an allergic reaction or a parasitic infection. The latest 6-part differential analyzers can also detect immature granulocytes, which are indicative of severe infections or certain hematological disorders, enabling earlier and more accurate diagnosis.
Today, artificial intelligence (AI) is being integrated into hematology analyzers. AI algorithms can analyze cell morphology with greater precision, identifying rare cell types and subtle abnormalities that may be missed by human observers. This technology has the potential to improve the detection of blood cancers and other hematological malignancies.
Microbiology focuses on identifying pathogens, and its evolution has been driven by the need for faster and more accurate diagnostic methods. Traditional methods, such as bacterial culture, were slow and often unreliable. Culturing a pathogen could take days or even weeks, during which the patient's condition might worsen. Microscopic examination of stained smears also had limitations, especially for fastidious organisms that were difficult to culture or detect.

Real-Time Polymerase Chain Reaction (RT-PCR) has transformed microbiology diagnostics. By amplifying the genetic material of pathogens, RT-PCR can detect even minute amounts of bacteria, viruses, or fungi in a matter of hours. During the COVID-19 pandemic, RT-PCR became the gold standard for diagnosing SARS-CoV-2. The test works by using specific primers to target the viral RNA, and fluorescent probes that bind to the amplified DNA, emitting a signal that can be detected in real-time. This allows for both the detection and quantification of the virus, providing valuable information for patient management and disease surveillance.
Automated culture systems have also improved traditional microbiology testing. These systems can monitor the growth of cultures more precisely, reducing the need for manual observation and speeding up the identification process. However, molecular techniques like RT-PCR offer several advantages, including higher sensitivity, specificity, and the ability to detect pathogens that are difficult to culture.

Next-generation sequencing (NGS) is the next frontier in microbiology. NGS can sequence the entire genome of a pathogen, providing detailed information about its genetic makeup, including drug resistance genes and strain variations. This technology has the potential to revolutionize outbreak investigations, allowing for rapid identification of the source of infection and the development of targeted treatment strategies.
In conclusion, in vitro diagnostics have come a long way, transforming healthcare through continuous innovation. From the early days of basic chemical reactions to the current era of AI-powered molecular detection, each advancement has brought us closer to providing more accurate, timely, and personalized medical care. As technology continues to evolve, the future of in vitro diagnostics looks promising, with the potential to further improve patient outcomes and revolutionize the practice of medicine.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)
Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style
Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip
Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette
Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit
Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit
Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit
Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit
Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit
Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit
Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit
Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit
Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)
Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl
Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl
Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)
Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)
Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)
Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |