- Home
- Resource
- Explore & Learn
- The Quest for Standardization in Laboratory Medicine: Ensuring Accurate and Harmonized Results
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Standardization in laboratory medicine is a cornerstone for ensuring the reliability and comparability of diagnostic test results. In the complex landscape of in vitro diagnostics (IVD), where numerous devices and methodologies are employed across different laboratories, achieving harmonized results is both a scientific and clinical imperative. The variability in test outcomes can significantly impact patient care, leading to misdiagnoses and inappropriate treatment decisions. Therefore, the quest for standardization is not merely a technical pursuit but a critical pathway to enhancing patient safety and clinical efficacy.
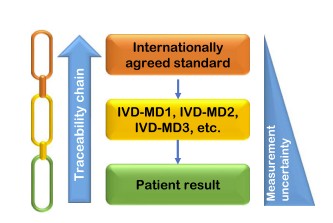 Fig.1 Schematic representation of metrological traceability approach to obtain harmonization of laboratory results. (Panteghini M., 2025)
Fig.1 Schematic representation of metrological traceability approach to obtain harmonization of laboratory results. (Panteghini M., 2025)
Metrological traceability serves as the bedrock for standardization in laboratory medicine. It involves anchoring measurements from IVD devices to higher-order references, ensuring that results are consistent and comparable across different platforms. The International Organization for Standardization (ISO) 17511:2020 standard provides a comprehensive framework for establishing metrological traceability. This standard outlines the requirements for calibration hierarchies, emphasizing the need for an unbroken chain of calibrations from international reference materials to end-user devices. By adhering to these guidelines, laboratories can achieve a high degree of accuracy and comparability in their test results.

Despite the clear guidelines provided by ISO 17511:2020, several challenges impede the successful implementation of metrological traceability. One significant issue is the non-commutability of reference materials. Reference materials must accurately mimic the properties of clinical samples to ensure that calibration biases are minimized. However, many reference materials currently in use have not been adequately assessed for commutability, leading to discrepancies in test results. For example, in the case of serum ferritin, a critical biomarker for iron deficiency, studies have shown that non-commutable reference materials can introduce significant biases, affecting the accuracy of measurements across different IVD devices.
Another challenge is the inadequate assessment of inter-assay variability. External Quality Assessment (EQA) programs, which are designed to evaluate the performance of IVD devices, often fall short in their ability to assess harmonization. Many EQA programs use non-commutable materials or rely on consensus values from peer groups, which do not provide a true measure of traceability. As a result, laboratories may meet regulatory requirements while still reporting biased results. This highlights the need for more robust EQA programs that incorporate commutable materials and reference measurement procedures (RMPs) to accurately assess the performance of IVD devices.
Achieving harmonized results also depends on the use of selective measurement methods. Selectivity ensures that the measurement process targets the specific analyte without interference from other components in the sample matrix. Non-selective methods, such as certain creatinine assays or albumin measurements, can introduce biases that significantly impact clinical decisions. For example, non-selective creatinine assays based on the alkaline picrate reaction can overestimate true serum creatinine concentrations due to the presence of pseudo-creatinine chromogens. This non-selectivity can lead to misclassification of patients with different grades of kidney function, potentially affecting their treatment plans.

In contrast, selective methods, such as enzymatic assays for creatinine or immunoturbidimetric assays for albumin, provide more accurate and harmonized results. These methods minimize interferences and ensure that measurements are consistent across different IVD devices. Therefore, laboratory professionals must prioritize the adoption of selective measurement methods to achieve the highest possible accuracy and comparability in their test results.

To address the challenges in implementing metrological traceability and achieving standardization, several practical solutions can be employed. First, reference material providers must ensure that reference materials are commutable and suitable for use in calibration hierarchies. This requires rigorous assessment of reference materials for commutability, as recommended by the IFCC Working Group on Commutability in Metrological Traceability. By using commutable reference materials, laboratories can minimize calibration biases and achieve more accurate results.
Second, EQA programs must be improved to better assess the performance of IVD devices in terms of metrological traceability. This involves incorporating commutable materials and using reference measurement procedures for value assignment. Collaboration between EQA organizers and reference material providers can help develop sustainable strategies for harmonization. For example, the use of commutable serum samples in EQA programs can provide a more accurate assessment of inter-assay variability and help identify areas for improvement.
Third, laboratory professionals must take responsibility for adopting selective measurement methods. International professional bodies should advocate for the use of selective assays through education and training programs. By prioritizing selective methods, laboratories can ensure that their test results are accurate and harmonized, providing a solid basis for clinical decision-making.
The case of serum ferritin measurements illustrates the complexities and challenges in achieving standardization. Ferritin is a critical biomarker for diagnosing iron deficiency, and clinical practice guidelines advocate specific decision thresholds for its measurement. However, studies have shown significant discrepancies in ferritin measurements across different IVD devices, highlighting the need for harmonization.
The calibration hierarchy for ferritin measurements involves international conventional calibrators (ICCs) defined by international protocols. However, the existence of multiple calibration hierarchies has introduced biases. For example, the transition from the first ICC (WHO IS code 80/602) to subsequent ones (e.g., WHO IS code 80/578) introduced a bias of approximately 5-10%. This bias is transferred to commercial immunoassays if different manufacturers use different calibration hierarchies.

Moreover, none of the WHO International Standards (ISs) for ferritin were originally assessed for commutability, potentially contributing to the observed inter-assay variability. A pilot study using a commutable serum sample revealed excessive inter-method differences, indicating that traceability claims by manufacturers did not lead to acceptable harmonization. This highlights the need for more robust EQA programs and the use of commutable reference materials to ensure accurate and harmonized ferritin measurements.
Standardization in laboratory medicine is essential for ensuring the accuracy and comparability of diagnostic test results. Achieving harmonized results requires a multi-faceted approach involving metrological traceability, commutable reference materials, robust EQA programs, and selective measurement methods. By addressing these aspects, stakeholders in the IVD industry can work towards achieving the highest possible standards of accuracy and comparability in laboratory medicine. The success of standardization efforts depends on the collective responsibility of all involved parties to uphold rigorous standards and continuously improve the quality of diagnostic testing.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |