- Home
- Resource
- Explore & Learn
- The Power of External Quality Assessment: A Deep Dive into EQA and Its Benefits for Medical Laboratories
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
External Quality Assessment (EQA) is an essential component of modern medical laboratory practice, providing a robust framework for evaluating and enhancing the accuracy and reliability of diagnostic testing. EQA programs, often referred to as proficiency testing, involve the distribution of standardized samples to participating laboratories, which are then analyzed using routine procedures. The results are compared across participants to identify discrepancies, assess performance, and provide actionable feedback. This process is vital for ensuring that laboratory results are consistent, accurate, and clinically relevant.
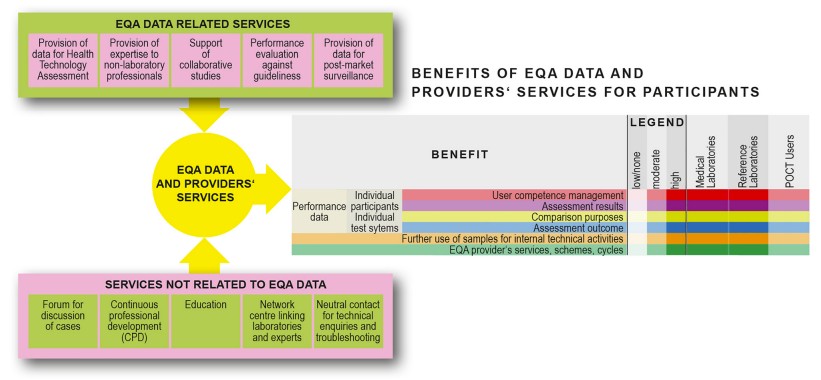 Fig.1 Benefits of EQA for participants. (Buchta C., et al., 2025)
Fig.1 Benefits of EQA for participants. (Buchta C., et al., 2025)

EQA is not merely a voluntary practice; it is a regulatory requirement for medical laboratories. International standards such as ISO 15189:2022 mandate that laboratories participate in interlaboratory comparisons through EQA programs. These standards emphasize the importance of using EQA results to improve future performance and correct past errors. Compliance with ISO 15189:2022 ensures that laboratories meet stringent quality requirements, thereby enhancing their credibility and reliability. Accreditation bodies often use EQA performance as a key indicator of a laboratory's competence, making it an essential tool for maintaining regulatory approval.
One of the primary benefits of EQA is its role in the verification and validation of In Vitro Diagnostic Medical Devices (IVD-MDs). Laboratories can use EQA samples to test the performance of these devices, ensuring they meet the manufacturer's specifications. This verification process is crucial for maintaining the accuracy and reliability of diagnostic tests, especially with the rapid advancement of new technologies. EQA programs provide an independent assessment of IVD-MDs, helping laboratories identify any deviations or errors in measurement, thereby ensuring that these devices perform as intended.


EQA plays a pivotal role in harmonizing laboratory practices globally. By comparing results across different laboratories and regions, EQA helps identify variations and inconsistencies in testing methods. This information is invaluable for developing standardized protocols and guidelines, ensuring that laboratories worldwide produce comparable and reliable results. Harmonization is particularly important in the context of global health challenges, where consistent diagnostic data is crucial for effective public health responses. For instance, during the COVID-19 pandemic, EQA programs were instrumental in ensuring that diagnostic tests for the virus were accurate and reliable across different laboratories.
EQA programs offer significant opportunities for the training and professional development of laboratory staff. Participation in EQA challenges exposes staff members to a variety of samples and testing methods, enhancing their skills and competence. EQA results can also be used to identify knowledge gaps and areas for improvement, leading to targeted training initiatives and better overall performance. This continuous professional development is essential for maintaining high standards of quality and ensuring that laboratory staff are well-equipped to handle emerging challenges in diagnostic testing.

EQA results are instrumental in estimating measurement uncertainty (MU), a critical factor in evaluating the quality of laboratory results. By comparing results from multiple laboratories, EQA programs can provide insights into the variability and reliability of different testing methods. This information helps laboratories refine their testing procedures, reduce uncertainty, and improve the overall quality of their results. For example, studies have shown that an analytical bias of 2% can result in a doubling of false positives in critical tests such as HbA1c and cholesterol screening. EQA programs help laboratories identify and correct such biases, thereby enhancing the accuracy and reliability of their diagnostic tests.
Medical Laboratories
For medical laboratories, EQA is essential for ensuring the accuracy and reliability of diagnostic tests. Participation in EQA programs helps laboratories identify and correct errors, validate their testing methods, and demonstrate their competence to regulatory bodies and healthcare providers. EQA also provides valuable data for continuous quality improvement, helping laboratories stay up-to-date with the latest advancements and best practices in the field.
Infection Diagnostics
Infection diagnostics, including bacteriology, virology, and parasitology, pose unique challenges due to the diversity and complexity of pathogens. EQA programs designed for these disciplines help laboratories detect and identify rare or exotic pathogens, ensuring that they are prepared to handle a wide range of infectious diseases. EQA also helps laboratories maintain high standards of accuracy and reliability in their diagnostic tests, which is crucial for effective infection control and public health.
Histoand Molecular Pathology Laboratories
Histoand molecular pathology laboratories play a crucial role in personalized medicine, providing detailed analyses of tissue samples and genetic markers. EQA programs for these laboratories help ensure the accuracy and reliability of their results, contributing to better patient outcomes. EQA also supports the development and validation of new biomarkers and testing methods, helping laboratories stay at the forefront of scientific advancements.
Point-of-Care Testing (POCT) and Self-Testing Devices
POCT devices and self-testing kits are increasingly used in healthcare settings, providing rapid diagnostic results at the point of care. EQA programs for POCT and self-testing devices help ensure that these devices produce accurate and reliable results, even when used by non-laboratory personnel. EQA also provides valuable feedback to manufacturers, helping them improve the performance and user-friendliness of their devices.
As laboratory medicine continues to evolve, EQA programs must adapt to incorporate new technologies and testing methods. Advances in molecular diagnostics, digital pathology, and artificial intelligence are transforming the field, and EQA providers must stay ahead of these changes to ensure that their programs remain relevant and effective. This includes developing new EQA materials and challenges that reflect the latest advancements in diagnostic testing.
One of the key challenges in EQA is ensuring the commutability of EQA materials, meaning that the samples used in EQA programs should behave similarly to real patient samples. Non-commutable materials can lead to inaccurate comparisons and misleading results. EQA providers must also ensure that their programs meet metrological criteria, such as traceability to higher-order measurement procedures and the use of reference materials. These efforts help ensure that EQA results are reliable and meaningful.
The future of EQA lies in global harmonization and collaboration. By working together, EQA providers and laboratories can develop standardized protocols and guidelines that ensure consistent and reliable diagnostic results worldwide. This collaboration is essential for addressing global health challenges and ensuring that patients receive the best possible care, regardless of their location.
External Quality Assessment (EQA) is a cornerstone of modern laboratory medicine, providing critical support for the accuracy and reliability of diagnostic tests. From enhancing patient care to ensuring regulatory compliance, EQA offers a wide range of benefits for medical laboratories. As the field of laboratory medicine continues to evolve, EQA programs must adapt to incorporate new technologies and address emerging challenges. By staying at the forefront of these advancements, EQA providers can help laboratories maintain high standards of quality and contribute to the global harmonization of diagnostic practices. In an era where accurate and reliable diagnostic data are more important than ever, EQA remains an essential tool for ensuring excellence in laboratory medicine.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |