Polycystic ovary syndrome (PCOS) is a common and complex endocrine disorder characterized by a triad of hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. This resource outlines the integrated diagnostic pathway for PCOS, detailing the systematic evaluation of each diagnostic cornerstone through precise laboratory assessment. We will explore the key biomarkers, imaging criteria, and essential differential diagnoses required to confidently apply the Rotterdam criteria.
Overview of Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders affecting individuals of reproductive age, characterized by a combination of hyperandrogenism (clinical or biochemical), ovulatory dysfunction, and polycystic ovarian morphology. It represents a complex multisystem condition with significant implications for reproductive health (infertility, pregnancy complications), metabolic health (insulin resistance, increased risk of type 2 diabetes and cardiovascular disease), and psychological well-being. Diagnosis relies on established criteria, such as the Rotterdam criteria, which require the presence of at least two of the three key features after the exclusion of other underlying disorders.
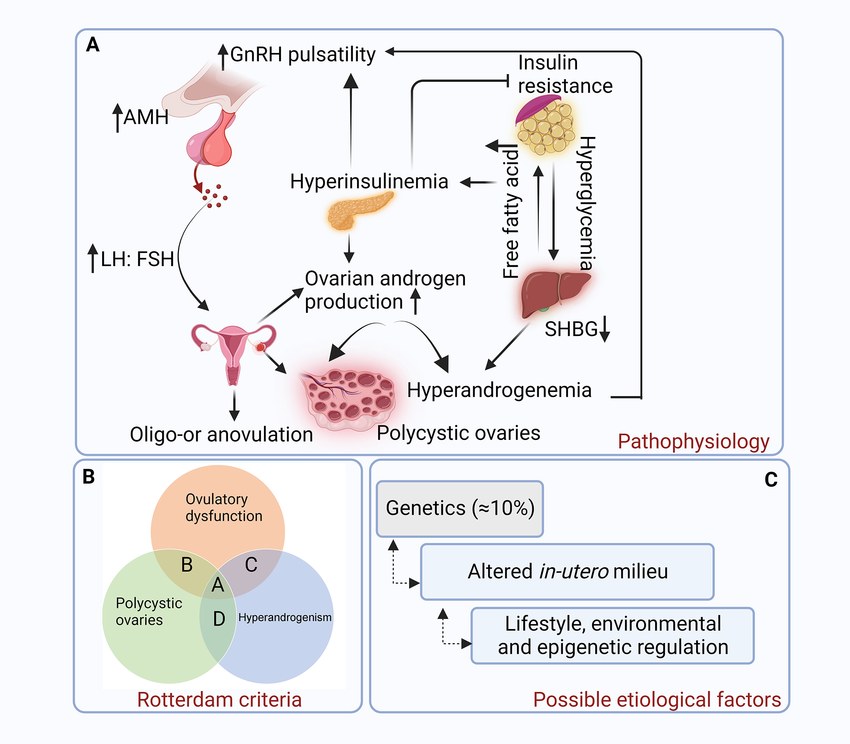 Fig.1 Pathophysiology of polycystic ovarian syndrome (PCOS). (Khatun, Masuma, et al., 2024)
Fig.1 Pathophysiology of polycystic ovarian syndrome (PCOS). (Khatun, Masuma, et al., 2024)
Cornerstone 1: Assessing Androgen Excess
Androgen excess is the most specific and central biochemical hallmark of PCOS. A thorough evaluation is crucial, as it distinguishes PCOS from other causes of anovulation and involves both clinical signs and definitive laboratory confirmation. Accurate assessment hinges on precise measurement of key hormones to document biochemical hyperandrogenism, which is required for diagnosis in patients without clear clinical signs.
Clinical Assessment of Hyperandrogenism
The initial evaluation involves identifying clinical signs of excess male hormones. The most specific and common manifestation is hirsutism, typically assessed using a standardized scoring system like the Ferriman-Gallwey score. Other clinical signs include moderate to severe acne that is persistent and treatment-resistant, and androgenic alopecia (female-pattern hair loss). It is important to note that the absence of these signs does not rule out androgen excess, making laboratory testing essential.
Key Biomarkers for Laboratory Confirmation
Laboratory testing provides the objective evidence needed to confirm biochemical hyperandrogenism. The cornerstone test is the measurement of total testosterone, which requires a sensitive and accurate assay optimized for the female range. To increase diagnostic sensitivity, especially when total testosterone is borderline, measurement of sex hormone-binding globulin (SHBG) is recommended to calculate the free androgen index (FAI). As an adjunct, dehydroepiandrosterone sulfate (DHEA-S) can be measured to assess the adrenal gland's contribution to the androgen pool.
Critical Step: Exclusion of Other Androgen-Excess Disorders
Before attributing androgen excess to PCOS, it is imperative to exclude other endocrine disorders. This differential diagnosis includes non-classic congenital adrenal hyperplasia (NCCAH), typically screened for with a morning 17-hydroxyprogesterone (17-OHP) level, as well as androgen-secreting tumors, Cushing's syndrome, and thyroid dysfunction. This exclusionary process ensures diagnostic accuracy and appropriate management.
Cornerstone 2: Documenting Ovulatory Dysfunction
Ovulatory dysfunction, manifesting as irregular or absent menstrual cycles, is a fundamental feature of PCOS and a major cause of infertility in affected individuals. Objective documentation is essential for diagnosis, especially in patients without clear hyperandrogenism. This assessment moves beyond patient history to include hormonal biomarkers that provide direct evidence of anovulation or infrequent ovulation.
Clinical Assessment: Menstrual History
The primary and most accessible indicator is the patient's menstrual history. Oligomenorrhea, defined as fewer than eight menstrual cycles per year, or amenorrhea, the absence of menstruation for three or more consecutive months, strongly suggests chronic anovulation. A detailed history is the critical first step that triggers further investigation.
Hormonal Biomarkers for Objective Confirmation
While a suggestive menstrual history is the clinical starting point, hormonal biomarkers provide the definitive biochemical proof of ovulatory dysfunction. The gold standard is the measurement of mid-luteal phase serum progesterone, where a level above a defined cutoff confirms ovulation has occurred, and persistently low levels confirm anovulation. As a supportive method, tracking basal body temperature (BBT) can show a characteristic biphasic pattern indicating ovulation, though it is less definitive than direct hormonal measurement.
The Link to Ovarian Reserve: Role of AMH
While not a direct marker of ovulation, anti-Müllerian hormone (AMH) provides valuable context. In PCOS, markedly elevated AMH levels, produced by the excessive number of small antral follicles, are strongly correlated with oligo-anovulation. High AMH may suppress follicular sensitivity to FSH, contributing to ovulatory dysfunction, thereby linking this cornerstone to the next (polycystic ovarian morphology).
Cornerstone 3: Evaluating Ovarian Morphology (PCOM)
The assessment of polycystic ovarian morphology (PCOM) is a key pillar in the diagnosis of PCOS, providing direct visual or biochemical evidence of the characteristic ovarian dysfunction. This evaluation traditionally relies on imaging but is increasingly supported by a highly correlated serum biomarker, offering a more accessible and objective alternative in many clinical settings.
- Transvaginal Ultrasound (TVUS): Transvaginal ultrasound is the imaging standard for PCOM, defined by specific morphologic criteria. Diagnosis requires either an increased antral follicle count (AFC≥20, follicles 2-9 mm) or enlarged ovarian volume (≥10 mL) per ovary, with absence of a dominant follicle. Result accuracy depends on operator skill and high-resolution equipment.
- Anti-Müllerian Hormone (AMH): AMH is a robust biochemical surrogate marker for PCOM, as it is secreted by small antral follicles. Elevated serum AMH levels strongly correlate with high AFC on ultrasound, with proposed diagnostic cutoffs. This provides a valuable objective measure when ultrasound is unavailable, suboptimal, or in adolescent patients.
IVD Products for Polycystic Ovary Syndrome (PCOS)
Alta DiagnoTech provides a comprehensive portfolio of in vitro diagnostic (IVD) solutions to support the precise and integrated diagnosis of polycystic ovary syndrome (PCOS). Our assays are designed to address each cornerstone of the Rotterdam criteria, from confirming biochemical hyperandrogenism and documenting ovulatory dysfunction to assessing ovarian reserve. These reliable tools deliver the accurate, actionable results necessary for differential diagnosis and informed patient management. If you have related needs, please feel free to contact us for more information or product support.
| Product Name |
Technology |
Application |
| Total Testosterone Immunoassay Kit |
Chemiluminescent Immunoassay (CLIA) |
Accurate quantification of serum total testosterone, the primary biomarker for confirming biochemical hyperandrogenism in PCOS. |
| SHBG (Sex Hormone-Binding Globulin) Assay Kit |
CLIA / Immunoturbidimetric |
Measurement of SHBG levels for calculating the Free Androgen Index (FAI), enhancing diagnostic sensitivity for hyperandrogenism. |
| Anti-Müllerian Hormone (AMH) ELISA Kit |
Enzyme-Linked Immunosorbent Assay (ELISA) |
Quantitative measurement of serum AMH as a biochemical surrogate marker for polycystic ovarian morphology (PCOM) and assessment of ovarian reserve. |
| Progesterone CLIA Kit |
Chemiluminescent Immunoassay (CLIA) |
Measurement of serum progesterone levels to objectively confirm ovulation (mid-luteal phase) or document anovulation. |
| 17-Hydroxyprogesterone (17-OHP) Assay Kit |
CLIA / ELISA |
Critical for the differential diagnosis to rule out non-classic congenital adrenal hyperplasia (NCCAH), a key mimic of PCOS. |
| Thyroid & Prolactin Screening Panel |
CLIA / ECLIA |
Simultaneous measurement of TSH and prolactin to exclude other common endocrine causes of menstrual irregularity and anovulation. |
| DHEA-Sulfate (DHEA-S) Immunoassay Kit |
CLIA |
Adjunctive test to assess adrenal androgen contribution in the evaluation of hyperandrogenism. |
| Insulin & Glucose Metabolism Panel |
CLIA / Colorimetric Enzymatic |
Includes insulin and glucose assays to assess insulin resistance and metabolic health in diagnosed PCOS patients, guiding long-term management. |
Reference
- Khatun, Masuma, et al. "Induced pluripotent stem cells as a possible approach for exploring the pathophysiology of Polycystic Ovary Syndrome (PCOS)." Stem cell reviews and reports 20.1 (2024): 67-87.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Pathophysiology of polycystic ovarian syndrome (PCOS). (Khatun, Masuma, et al., 2024)
Fig.1 Pathophysiology of polycystic ovarian syndrome (PCOS). (Khatun, Masuma, et al., 2024)