- Home
- Resource
- Explore & Learn
- The Metabolomics Revolution: Unveiling the Secrets of Disease through Biomarkers
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Metabolomics is rapidly emerging as a transformative field in biomedical research and clinical diagnostics. By providing a detailed view of the metabolic processes occurring in the human body, this technology offers new opportunities for the identification of biomarkers that could be pivotal in diagnosing, predicting, and monitoring diseases. In contrast to genomics and proteomics, metabolomics directly measures the small molecules, or metabolites, in biological samples, reflecting the end-products of the biochemical pathways driven by both genetics and environmental influences.
As the demand for personalized medicine grows, metabolomics promises to enhance disease detection, provide insights into treatment efficacy, and ultimately improve patient outcomes. However, despite its potential, the integration of metabolomics into clinical practice faces significant challenges. From the complexities of sample collection and analysis to the rigorous validation requirements for biomarkers, understanding these hurdles is key to unlocking the full potential of metabolomics in modern healthcare.
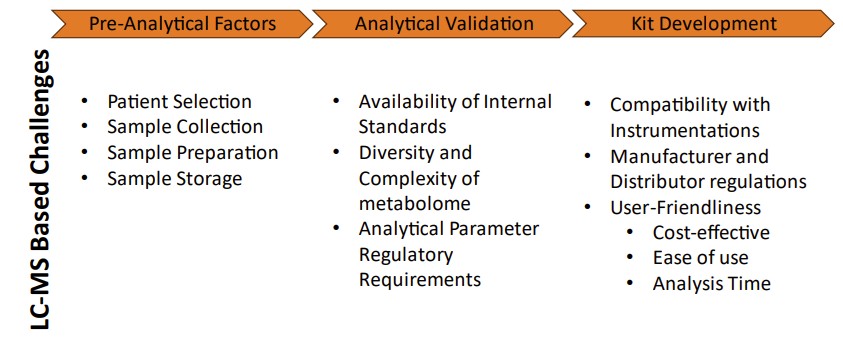 Fig.1 Overview of challenges in an LC-MS-based metabolomics biomarker validation pipeline. (Li S., et al., 2024)
Fig.1 Overview of challenges in an LC-MS-based metabolomics biomarker validation pipeline. (Li S., et al., 2024)
At its core, metabolomics is the study of small molecules in biological systems, typically those with a molecular weight of less than 1,500 Da. These metabolites include sugars, amino acids, lipids, fatty acids, and nucleotides, which collectively reflect the biochemical state of an organism. Since metabolites are influenced by genetic factors, environmental exposures, and lifestyle choices, they offer a dynamic snapshot of an individual's metabolic health.
One of the main advantages of metabolomics is its ability to provide real-time data on the biochemical status of the body, making it an invaluable tool for disease monitoring. By examining metabolic alterations, researchers and clinicians can track the progression of diseases, such as cancer, cardiovascular disorders, and neurological conditions, and evaluate the effects of various treatments.
Metabolomics often utilizes advanced analytical techniques, with liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) being one of the most commonly used methods. This approach allows researchers to separate, identify, and quantify metabolites in complex biological samples with high sensitivity and specificity. Despite the advances in technology, challenges in metabolite identification, data interpretation, and validation remain significant barriers to widespread clinical adoption.
Biomarkers are biological indicators that can provide critical insights into the presence, progression, or therapeutic response of a disease. The discovery and validation of reliable biomarkers are crucial for early disease detection, personalized treatment strategies, and monitoring disease activity. Traditional biomarkers, such as proteins and nucleic acids, have been instrumental in diagnostics, but their utility is often limited by issues such as low sensitivity, specificity, and the ability to capture the full complexity of diseases.
Metabolomics offers a unique advantage in this regard by enabling the identification of a broad spectrum of metabolites that may serve as novel biomarkers. These biomarkers can provide a more accurate reflection of the physiological state of an individual, as metabolites directly reflect the functional activity of cells and tissues. For example, in the case of cancer, changes in metabolic pathways, such as increased glycolysis (the Warburg effect), have been observed in various types of tumors. By identifying these alterations through metabolomics, new diagnostic and prognostic biomarkers can be discovered.
Moreover, metabolomics can identify panels of biomarkers, which can increase diagnostic accuracy. Biomarker panels, composed of multiple metabolites, may offer superior sensitivity and specificity compared to single markers, making them more reliable in distinguishing between healthy and diseased states. For example, the combination of lipids, fatty acids, and amino acids in a biomarker panel could be used to detect early-stage cardiovascular diseases, where individual markers alone may not be sufficient.
Before the analysis of metabolites can take place, proper sample collection and handling are critical to ensuring accurate results. Pre-analytical factors—such as patient selection, sample collection, storage, and preparation—can significantly influence the outcomes of metabolomic studies. Without rigorous standard operating procedures (SOPs), metabolite degradation or variations introduced during handling can lead to inconsistencies in measurements, undermining the reliability of results.
Patient selection is one of the first steps in mitigating pre-analytical challenges. Given that the metabolome is highly influenced by environmental factors such as diet, smoking, and medications, it is essential to minimize these confounders during sample collection. For instance, a study of smoking-induced metabolic changes found that 168 metabolites were significantly altered due to smoking, and these changes were associated with body mass index (BMI). Other factors, such as age, sex, and comorbidities, can also affect metabolite levels and must be taken into account when selecting patient cohorts for metabolomic studies.
Sample collection and storage are equally important. Metabolites are often sensitive to temperature, pH, and storage conditions. For example, improper anticoagulant use during blood collection can lead to the alteration of lipid profiles, while hemolysis (the rupture of red blood cells) can introduce additional metabolites that obscure the true metabolic profile. To prevent these issues, standardized protocols for sample collection and handling should be established and followed meticulously.
Furthermore, many metabolites fluctuate throughout the day, influenced by circadian rhythms or postprandial states (whether the person has eaten or not). This necessitates careful timing of sample collection to avoid variations that could skew results. For instance, metabolites such as fatty acids, lipids, and amino acids display time-of-day variations, which can be influenced by factors such as food intake and sleep patterns.
Once proper sample collection and handling procedures are in place, the next step is analytical validation. Analytical validation ensures that the chosen method for metabolite measurement is reliable, accurate, and reproducible. In the case of metabolomics, LC-MS/MS is the gold standard for analyzing metabolites, offering high sensitivity, specificity, and the ability to perform both targeted and untargeted analysis.
Several key parameters must be validated to ensure the robustness of the analytical method. These include sensitivity (the ability to detect low concentrations of metabolites), specificity (the ability to distinguish the metabolite of interest from other compounds), and accuracy (the degree to which the measured value reflects the true value). The calibration curve used in LC-MS/MS analysis is critical for establishing the relationship between metabolite concentration and the instrument's response, ensuring that measurements are both reliable and reproducible.
Additionally, stability testing is essential to account for factors such as sample storage conditions and freeze-thaw cycles, which can affect metabolite levels. Regulatory guidelines, such as those from the International Council for Harmonisation (ICH), provide specific criteria for analytical method validation, including the evaluation of matrix effects (how sample components may interfere with metabolite detection) and the stability of analytes in different storage conditions.
Despite these rigorous validation steps, the complexity of the metabolome presents significant challenges. The metabolome encompasses a wide variety of metabolites with vastly different chemical properties, which can span several orders of magnitude in concentration. This broad range poses difficulties in ensuring that the method can accurately measure all metabolites of interest. Furthermore, metabolites may interact with one another, complicating their measurement and analysis. These challenges highlight the need for advanced analytical methods that can handle the diversity and complexity of the metabolome.
For metabolomics-based biomarkers to transition from research to clinical practice, it is essential to develop commercial diagnostic kits that can be used across a wide range of laboratories. This process involves several steps, including the standardization of analytical methods, ensuring compatibility with existing laboratory instruments, and meeting regulatory requirements for clinical use.
One of the main challenges in developing metabolomics-based kits is ensuring that they are compatible with different types of mass spectrometers and other laboratory equipment. Various mass spectrometer models may have differences in sensitivity, ionization techniques, and fragmentation capabilities, which can impact the accuracy of metabolite measurements. To overcome this issue, kit manufacturers must ensure that their products are compatible with a broad range of instruments or develop strategies to modify the kits for specific laboratory setups.
In addition to technical challenges, regulatory hurdles must be overcome before metabolomics-based diagnostic kits can be widely used in clinical settings. Manufacturers must comply with stringent regulatory standards from agencies such as the U.S. Food and Drug Administration (FDA) or Health Canada. This includes providing detailed documentation on the kit's intended use, methodology, limitations, and instructions for use. Clinical validation studies must also be conducted to demonstrate that the kit provides accurate and reliable results across different patient populations.
Commercial kits such as Biocrates' AbsoluteIDQ P400 HR Kit and OwlMetabolomics' diagnostic tests for fatty liver disease have demonstrated the potential for metabolomics in clinical diagnostics. However, the road to widespread adoption is still fraught with challenges related to cost, complexity, and the need for ongoing validation.
Metabolomics holds tremendous promise for transforming disease diagnosis, prognosis, and treatment in the era of precision medicine. By providing a comprehensive and dynamic view of an individual's metabolic state, metabolomics has the potential to identify novel biomarkers that can detect diseases at earlier stages, predict treatment outcomes, and monitor disease progression. However, significant challenges remain, particularly in terms of sample collection, analytical validation, and the development of commercially viable diagnostic kits.
As technology advances and the understanding of the metabolome deepens, the integration of metabolomics into clinical practice will become increasingly feasible. The development of standardized protocols, improved analytical methods, and commercial kits will be key to realizing the full potential of metabolomics in precision medicine. Through continued research and innovation, the metabolomics revolution has the potential to change the way we diagnose, treat, and manage diseases, ultimately improving patient outcomes across the globe.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |