- Home
- Resource
- Explore & Learn
- The Hidden Dangers of Mixing and Matching Blood Collection Systems
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In the realm of in vitro diagnostics, the preanalytical phase, particularly blood collection, lays the groundwork for accurate and reliable diagnostic outcomes. Venous blood collection systems (VBCSs) are pivotal tools used to obtain blood samples for a myriad of tests, ranging from routine blood counts to complex biochemical analyses. These systems, though seemingly simple, are sophisticated assemblies of components that must work in harmony to ensure sample integrity and safety. However, the practice of mixing and matching components from different manufacturers—referred to as combined systems—poses significant risks that can compromise diagnostic accuracy and patient safety.
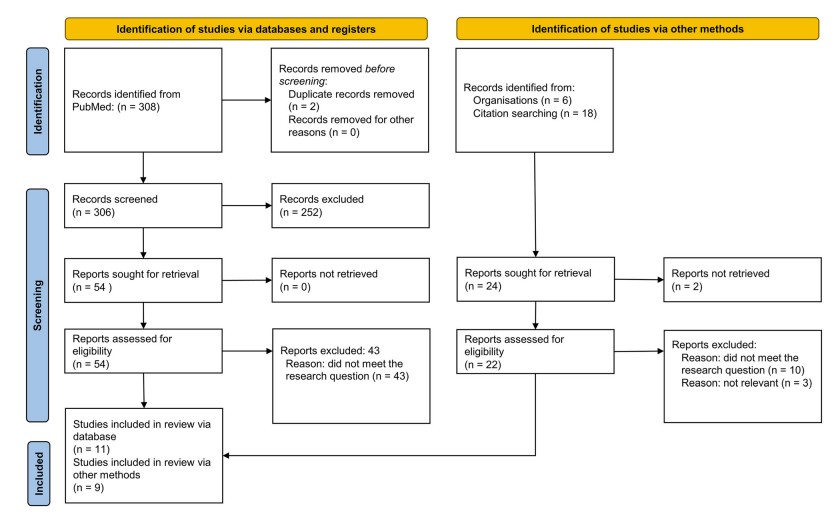 Fig.1 Flowchart of the systematic review according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) model. (Rigoni M., et al., 2025)
Fig.1 Flowchart of the systematic review according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) model. (Rigoni M., et al., 2025)
Integrated VBCSs are meticulously designed and validated by manufacturers to ensure that all components, including blood collection needles, tube holders, and evacuated blood collection tubes, work seamlessly together. These systems undergo rigorous testing to guarantee compatibility, performance, and safety. For instance, manufacturers must adhere to stringent regulatory standards such as the European Union's Medical Devices Regulation (MDR) 2017/745 and the In Vitro Diagnostic Medical Devices Regulation (IVDR) 2017/746, which mandate comprehensive validation processes to ensure that integrated systems meet specific performance criteria.
In contrast, combined VBCSs, which are assembled from components sourced from different manufacturers, lack this level of validation. Without a comprehensive assessment of compatibility, these systems can introduce a range of issues that affect both the quality of the blood sample and the safety of the procedure. For example, CLSI standards and WHO guidelines highlight that combining components from different manufacturers can lead to technical problems such as hemolysis, inadequate tube filling, and even disengagement of the needle from the tube holder. These issues not only compromise the integrity of the sample but also pose risks to patient and operator safety.
The analytical performance of blood collection systems is highly dependent on the consistency and compatibility of the components. Different manufacturers may use varying materials, additives, and lubricants in their blood collection tubes, which can interfere with test results. For instance, tubes containing separator gels have been shown to produce falsely reduced results due to interactions between the gel and the blood sample. Additionally, the presence of surfactants, stopper materials, and other additives can introduce variability that affects the accuracy of diagnostic assays.
Given the potential for variability, laboratories must undertake local validation of combined systems to ensure their suitability for use. This involves rigorous testing to verify that the components work together without compromising the quality of the sample or the accuracy of the test results. However, this process is resource-intensive and often overlooked, leading to the use of systems that may not meet the required standards.
The use of combined VBCSs can introduce technical and procedural risks that directly impact patient safety. For example, the combination of components from different manufacturers can lead to issues such as hemolysis, which can invalidate test results and necessitate repeat sampling. Additionally, the efficacy of safety mechanisms, such as needle guards, can be compromised when components are not fully compatible, increasing the risk of needlestick injuries.
The potential for errors in sample collection and handling is heightened when using combined systems. For instance, the use of tubes with different color codes can lead to confusion and errors in sample collection order, impacting both patient safety and diagnostic outcomes. Furthermore, the lack of validation for combined systems means that healthcare professionals cannot be certain of their safety and efficacy, leading to increased risks for both patients and operators.
While the initial cost of purchasing separate components for combined systems may appear lower, the long-term economic impact can be significant. Laboratories must invest in validation processes to ensure the compatibility and performance of these systems, which can be both time-consuming and costly. Additionally, the risk of errors and the need for repeat testing can further increase healthcare costs.
The use of combined systems can lead to hidden costs that are often overlooked. For example, the need for repeat sampling due to compromised sample quality can increase the overall cost of diagnosis. Moreover, the potential for errors and adverse events can lead to increased liability and the need for additional training and resources to manage these risks.

The European Union's regulatory framework provides clear guidelines for the validation and verification of integrated VBCSs. Manufacturers are required to ensure that all components of an integrated system are fully compatible and safe for use. However, the situation is less clear for combined systems, which are often assembled by clinical laboratories without comprehensive validation. While Article 22 of the EU Regulation 2017/745 outlines responsibilities for those who place combined systems on the market, there is no explicit guidance for laboratories that assemble these systems for internal use.

Given the potential risks associated with combined VBCSs, clinical laboratories are advised to prioritize the use of fully integrated systems. When considering the purchase of a new system or components, laboratories should conduct thorough validation and verification processes to ensure compatibility and performance. This includes evaluating the analytical performance of the system, verifying the safety mechanisms, and ensuring that all components work together seamlessly.
The current evidence suggests that combined VBCSs are not equivalent to integrated systems in terms of safety and efficacy. There is a clear need for more comparative studies to quantify these differences and provide a stronger basis for decision-making. Additionally, future research should focus on developing standardized validation protocols for combined systems to ensure their safe and effective use in clinical settings.
To address the challenges posed by combined systems, there is a need for the development of standardized validation protocols. These protocols should include comprehensive testing of analytical performance, safety mechanisms, and overall system compatibility. By establishing clear guidelines for validation, laboratories can better ensure the safety and efficacy of the blood collection systems they use.
The choice of blood collection system is a critical factor in ensuring the accuracy and safety of diagnostic tests. While integrated VBCSs offer a validated and reliable solution, combined systems come with significant risks that can compromise patient care. By prioritizing the use of integrated systems and investing in thorough validation processes, clinical laboratories can help ensure the highest possible standards of diagnostic accuracy and patient safety. As the field of in vitro diagnostics continues to evolve, it is essential that we remain vigilant in our efforts to maintain the integrity of the diagnostic process and protect the well-being of patients and healthcare professionals alike.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |