- Home
- Resource
- Explore & Learn
- The Future of Medical Diagnostics: How SERS Technology is Revolutionizing Disease Detection
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Surface-Enhanced Raman Scattering (SERS) is rapidly emerging as a groundbreaking technology in medical diagnostics, delivering unparalleled sensitivity and multiplex detection capabilities. Traditional diagnostic methods, such as electrochemiluminescence (ECL), enzyme-linked immunosorbent assay (ELISA), and fluorescence detection, often face limitations in detecting biomarkers at low concentrations. In contrast, SERS capitalizes on the localized surface plasmon resonance (LSPR) effect to significantly amplify Raman signals, allowing for the detection of biomarkers at concentrations as low as the single-molecule level. This innovative technology holds the potential to transform the detection of diseases like cancer and infectious diseases by providing faster, more accurate, and highly sensitive diagnostic tools.
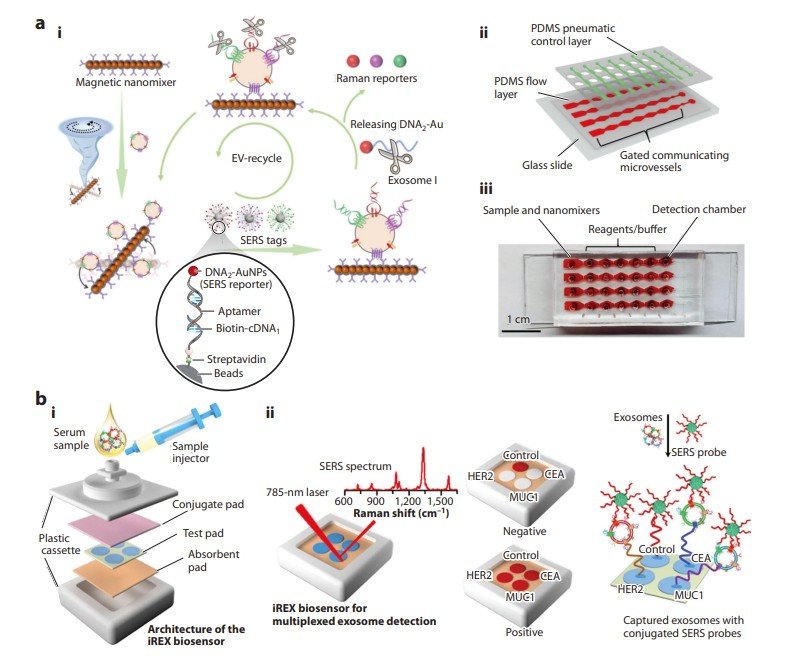 Fig.1 Application of SERS-based diagnostics to cancer biomarkers in liquid biopsy. (Joung Y., et al., 2025)
Fig.1 Application of SERS-based diagnostics to cancer biomarkers in liquid biopsy. (Joung Y., et al., 2025)
SERS operates by enhancing the Raman scattering signals of molecules through the interaction with metallic nanostructures, typically composed of gold or silver. The LSPR effect, which occurs when incident light interacts with free electrons on the surface of these nanostructures, significantly amplifies the electromagnetic field, leading to an enhanced Raman signal. This enhancement allows SERS to detect biomarkers at extremely low concentrations, overcoming the limitations of traditional methods.
Cancer remains one of the most challenging diseases to diagnose and treat, often requiring invasive procedures like tissue biopsy. Liquid biopsy, which involves analyzing biomarkers in biofluids such as blood, offers a non-invasive alternative. SERS technology is playing a crucial role in advancing liquid biopsy by enabling the detection of biomarkers like extracellular vesicles (EVs), circulating tumor cells (CTCs), and circulating tumor DNA (ctDNA).

EVs, including exosomes, are small vesicles secreted by tumor cells and carry valuable information about cancer development and metastasis. SERS-based assays for EVs can be categorized into labeled and non-labeled techniques:
Non-labeled SERS Detection
This technique directly analyzes clinical samples without labeling. Machine learning algorithms are used to interpret the complex SERS signals. For instance, Jalali et al. developed a SERS-based microfluidic chip combined with a convolutional neural network (CNN) to analyze the molecular profiles of single EVs.
Labeled SERS Detection
This involves using SERS nanotags that bind to specific proteins on the EV surface. For example, Zhang et al. developed a microfluidic chip that uses antibody-conjugated magnetic nanomixers to capture EVs and detect multiple protein biomarkers simultaneously.
CTCs are rare cells that detach from primary tumors and enter the bloodstream. Detecting CTCs is challenging due to their low concentration. SERS technology, combined with microfilters, has shown promise in isolating and detecting CTCs with high sensitivity. For example, Xu et al. used a microfilter embedded in a microfluidic channel to isolate CTCs and then applied SERS nanotags for detection.

ctDNA and ctRNA are fragments of genetic material released by tumors into the bloodstream. SERS-based assays have been developed to detect these biomarkers with high sensitivity. For instance, Jiang et al. developed an in situ SERS detection method for microRNAs (miRNAs) in exosomes, achieving a detection limit of 0.21 fM.
The COVID-19 pandemic highlighted the need for rapid, accurate, and sensitive diagnostic methods. While real-time polymerase chain reaction (RT-PCR) remains the gold standard, it requires extensive sample preparation and long processing times. SERS-based diagnostics offer a potential solution by providing faster results with high sensitivity.
SERS technology has been integrated into molecular diagnostic assays to reduce diagnostic times. For example, Yang et al. developed a SERS-based platform that combines a silver nanorod array substrate with a deep learning model to detect SARS-CoV-2 RNA in nasopharyngeal swabs. This method achieved an accuracy of 98.9% and significantly reduced the time required for diagnosis.
Lateral flow assays (LFAs) are widely used for point-of-care testing (POCT) due to their simplicity and speed. However, traditional LFAs have limitations in sensitivity. SERS-based LFAs offer a significant improvement by using SERS nanotags instead of traditional colorimetric indicators. For instance, Joung et al. developed a portable SERS-LFA system that achieved a detection limit of <5 PFU/mL for SARS-CoV-2, compared to 500 PFU/mL for commercial LFAs.
Despite the promising advancements, several challenges must be addressed to bring SERS technology from the laboratory to clinical practice:
Developing SERS substrates with consistent and controlled nanogap formation is crucial for reproducibility. Current methods often struggle with achieving uniformity, leading to variability in signal enhancement.
Embedding SERS detection into microdevices like LFAs and microfluidic channels requires further development. The integration must ensure reliable and consistent performance, especially in point-of-care settings.
Comparative studies with existing clinical methods and FDA approval are necessary for widespread adoption. Clinical trials must demonstrate that SERS-based diagnostics are not only more sensitive but also cost-effective and easy to use.
SERS technology is revolutionizing the field of in vitro diagnostics by offering unprecedented sensitivity and multiplex detection capabilities. Its applications in cancer and infectious disease diagnostics highlight its potential to transform clinical practice. While challenges remain, ongoing research and technological advancements are paving the way for SERS-based diagnostics to become a standard tool in healthcare. As we continue to push the boundaries of what is possible, SERS holds the promise of a future where diseases can be detected earlier, treated more effectively, and managed with greater precision.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |