- Home
- Resource
- Explore & Learn
- The Future of Diagnosing Urinary Tract Infections: A Leap Beyond Traditional Methods
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Urinary tract infections (UTIs) are one of the most prevalent bacterial infections globally, imposing a significant burden on public health. Traditional diagnostic methods, such as urinalysis and urine culture, have long been the mainstay of clinical practice. However, these methods are often time-consuming and may lack sensitivity, particularly in detecting low-level infections or fastidious organisms. The advent of novel diagnostic technologies is poised to transform the landscape of UTI diagnosis, offering faster, more accurate, and more comprehensive results. This article explores the latest advancements in UTI diagnostics, focusing on rapid molecular identification, next-generation sequencing (NGS), and rapid phenotypic susceptibility testing.
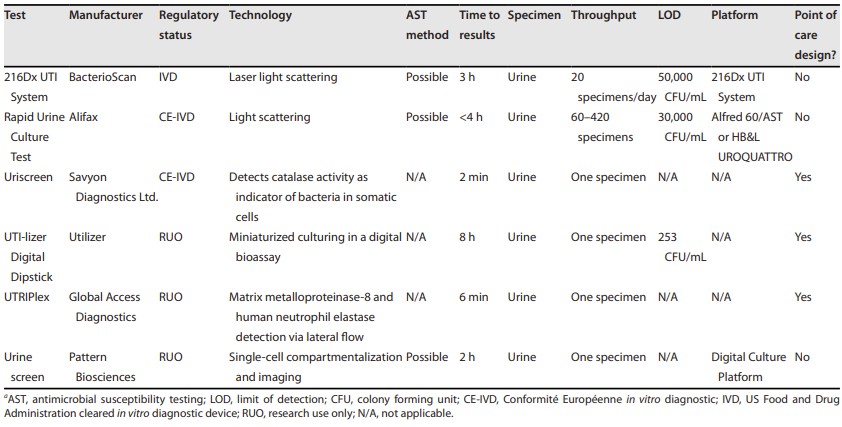 Fig.1 Summary of novel urine screening assays for prediction of bacteriuria. (Bermudez T., et al., 2025)
Fig.1 Summary of novel urine screening assays for prediction of bacteriuria. (Bermudez T., et al., 2025)
Traditional urine culture has been the cornerstone of UTI diagnosis since the 1950s. However, this method is not without its drawbacks. Urine cultures require a minimum bacterial concentration (typically 10^5 CFU/mL) to be considered positive, which may overlook low-level infections. Moreover, the process can take up to 48 hours, delaying targeted antibiotic therapy. Additionally, traditional cultures are performed under aerobic conditions, which may not capture fastidious or anaerobic organisms. Recent studies have shown that urine is not sterile, and many microorganisms previously thought to be contaminants may actually play a role in UTIs. These limitations highlight the need for more advanced diagnostic methods.
Rapid molecular identification methods represent a significant advancement in UTI diagnostics. These methods leverage nucleic acid amplification techniques to detect the genetic material of pathogens directly from urine samples. Multiplex PCR assays, for example, can identify multiple bacterial species and antimicrobial resistance genes simultaneously, providing results within hours rather than days. The Lodestar Dx UTI test uses loop-mediated amplification to detect six bacterial targets in urine within 30 minutes. Similarly, the Vivalytic UTI RUO Test is designed for point-of-care use and can identify 20 bacterial targets and eight resistance genes in under three hours. These technologies offer high sensitivity and specificity, making them ideal for rapid diagnosis and targeted treatment.

Next-generation sequencing (NGS) represents a paradigm shift in molecular diagnostics. NGS can sequence the entire genome of pathogens present in a urine sample, providing detailed information about the types and quantities of bacteria, fungi, and viruses. This technology can detect even low-level infections and identify organisms that are not cultivable by traditional methods. For instance, Illumina's Urinary Pathogen ID/AMR (UPIP) enrichment kit can detect 121 bacterial species, 3,728 antimicrobial resistance genes, 14 fungi, 4 parasites, and 35 viruses within 24 hours. Studies have shown that NGS can detect a significantly higher diversity of microorganisms compared to standard cultures, potentially improving diagnostic accuracy. NGS also allows for the detection of emerging uropathogens, such as Aerococcus urinae, Actinotignum schaalii, and Alloscardovia omnicolens, which are often missed by traditional cultures.
Rapid phenotypic susceptibility tests offer a significant improvement over traditional antimicrobial susceptibility testing. These methods measure the growth of bacteria in the presence of antibiotics, providing information about which drugs are effective against the infecting organism. Unlike traditional susceptibility testing, which can take several days, these new tests can yield results within hours. For example, the Astrego AST technology uses nanofluidics to measure bacterial growth in nanochannels, providing susceptibility results within 30 minutes. Similarly, the iFAST system measures changes in bacterial cell health using impedance flow cytometry, offering results in about five hours. These technologies enable clinicians to prescribe targeted antibiotics more quickly, reducing the risk of antibiotic resistance.
The implementation of novel diagnostic technologies has the potential to significantly improve patient outcomes. Rapid molecular identification and NGS can detect infections that traditional cultures miss, leading to more accurate diagnoses. Rapid phenotypic susceptibility tests can provide targeted antibiotic therapy more quickly, reducing the time patients spend on broad-spectrum antibiotics. This not only improves patient outcomes but also helps combat the growing problem of antibiotic resistance. Additionally, these technologies can reduce the time and resources required for diagnosis, potentially lowering healthcare costs.
While novel diagnostic technologies offer significant advantages, their implementation in clinical practice faces several challenges. One major issue is the need for validation and regulatory approval. Many of these tests are still in development or undergoing clinical trials, and their performance characteristics need to be thoroughly evaluated. Another challenge is the interpretation of results. Molecular diagnostics can detect a wide range of microorganisms, but determining which ones are clinically significant can be complex. For instance, detecting low-level bacteria in asymptomatic individuals may not necessarily indicate an active infection. Clinicians need guidelines to help them interpret these results and make informed treatment decisions.
The future of UTI diagnosis lies in the integration of advanced diagnostic technologies with clinical practice. These new tools have the potential to improve diagnostic accuracy, reduce the time to effective treatment, and minimize the overuse of antibiotics. However, their successful implementation will require collaboration between researchers, clinicians, and regulatory bodies to ensure that they are safe, effective, and clinically meaningful. As we move forward, it is crucial to continue researching the urinary microbiome and its role in health and disease. Understanding the complex interactions between different microorganisms and the host will help us develop more targeted diagnostic and therapeutic strategies. Additionally, ongoing education and training for healthcare providers will be essential to ensure that they can effectively use these new technologies and interpret their results.
The diagnosis of UTIs is on the cusp of a significant transformation, thanks to advancements in molecular diagnostics and next-generation sequencing. These new technologies offer the promise of faster, more accurate, and more comprehensive results, potentially improving patient outcomes and reducing the burden of UTIs on healthcare systems. While challenges remain, the future looks promising, and the integration of these innovations into clinical practice could mark a new era in the management of urinary tract infections.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |