Metrological traceability in laboratory medicine ensures that the measurement results for a clinical sample are consistent, accurate, and comparable across different laboratories and diagnostic systems. It is defined as an unbroken chain of measurements from the clinical sample result back to a reference standard. This chain links every measurement step to ensure that a diagnostic result is as accurate as possible. The integrity of this process relies heavily on the use of reference materials, measurement procedures, and the calibration of in vitro diagnostic medical devices (IVD-MDs).
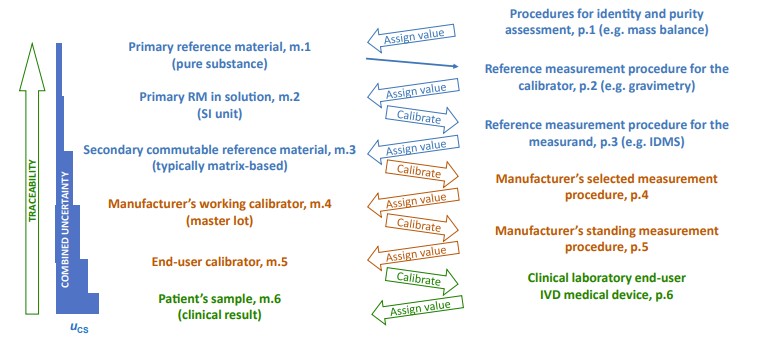 Fig.1 Metrological traceability as defined in ISO 17511:2020. (Greg M. W., et al., 2024)
Fig.1 Metrological traceability as defined in ISO 17511:2020. (Greg M. W., et al., 2024)
Calibration Hierarchy and Its Role in Traceability
The calibration hierarchy is the backbone of metrological traceability, as outlined in ISO 17511. At the top of this hierarchy are primary reference materials (RMs) or reference measurement procedures (RMPs). These standards serve as the most accurate measurements from which all others are derived. As measurements move through the chain, secondary RMs, IVD-MDs, and clinical laboratories take on progressively lower positions, each step introducing a level of uncertainty.
At every level of this hierarchy, there is an associated uncertainty. For example, when a clinical laboratory measures a sample using an IVD-MD, the uncertainty from the reference materials used to calibrate the IVD-MD accumulates, potentially affecting the final result. This combined uncertainty must be kept within acceptable limits to avoid errors in clinical diagnosis and treatment.
Analytical Performance Specifications (APS): The Foundation of Accuracy
Defining the Maximum Allowable Uncertainty (umaxCS)
To ensure clinical decisions are based on accurate and reliable test results, analytical performance specifications (APS) are established. These specifications define the maximum allowable uncertainty in clinical sample results, known as umaxCS. When clinical sample results exceed this allowable uncertainty, the diagnostic result may be deemed unfit for making medical decisions.
umaxCS serves as the threshold that ensures the clinical laboratory result is within a tolerable margin of error, and its value is derived from biological variability and the measurement uncertainty at each step in the calibration hierarchy. The goal is to minimize errors at each stage, from the calibration of the IVD-MD to the final patient result.
Importance of APS in Laboratory Medicine

APS for maximum allowable uncertainty plays a critical role in maintaining the accuracy of clinical tests. These specifications act as a quality control measure that ensures the uncertainty at each step in the calibration hierarchy is minimized to meet the umaxCS. By calculating the total uncertainty in the measurement process and allocating it properly across different stages, APS can help prevent errors in clinical diagnostics. The higher the APS, the greater the room for uncertainty, which could lead to misdiagnoses and improper treatments.
Estimating Uncertainty in Clinical Laboratories: Challenges and Solutions
Repeatability Uncertainty (uRw) in IVD-MDs
A key component of metrological traceability is repeatability uncertainty (uRw), which reflects the variability in test results when the same IVD-MD is used multiple times within the same laboratory. Repeatability accounts for sources of uncertainty inherent in the operation of diagnostic equipment, such as recalibrations, reagent lot changes, and other operational factors.
ISO TS 20914 provides practical guidance for estimating uRw by using internal quality control (IQC) data from the clinical laboratory. However, accurately capturing uRw requires sufficient data over an appropriate time interval. A major challenge is ensuring that the data used to estimate uRw is representative of the variability in the laboratory's operations. Without a sufficiently long time period, short-term variations may lead to an underestimation of uRw, which can compromise the assessment of diagnostic performance.
Impact of IQC Data and Reagent Lot Changes
One of the challenges in estimating uRw involves the frequent changes in reagent and calibrator lots. These changes can introduce biases that are not immediately detectable through traditional IQC measurements. As shown in real-world examples, shifts in IQC results without corresponding changes in clinical samples can significantly affect the repeatability of test results. The variability in IQC results across different reagent lots can lead to an underestimation of uncertainty if not handled properly.
To minimize these issues, it is essential to partition IQC results according to different reagent lots and calibrators and calculate the variability separately for each. Pooling data from multiple IQC lots and calibrator sets over an extended period helps to provide a more accurate estimate of uRw, thus improving the overall uncertainty calculation.
The Need for Extended Time Intervals in uRw Estimation
Short-term data analysis can be misleading, as laboratory conditions may vary over time. For instance, variability in measurements may increase as reagent lots and instrument calibrations change. A study analyzing the IQC data for serum creatinine measurements over a two-year period demonstrated that the coefficient of variation (CV) varied significantly depending on the time interval used for analysis. Shorter sampling periods led to different uRw estimates, illustrating the importance of accounting for long-term variability in uncertainty calculations.
The Role of Commutability in Reference Materials
What is Commutability?
Commutability is a crucial aspect of metrological traceability. It refers to the ability of a reference material (RM) to behave similarly to clinical samples when used for calibration. If a reference material is noncommutable, it may introduce a bias in the calibration process, leading to inaccurate test results. This bias is particularly concerning when a calibrator lot changes or when different IVD-MDs are used.
Noncommutability Bias and Its Impact
Noncommutability bias is the difference in performance between a reference material and clinical samples. If the RM behaves differently from the clinical samples, this difference can lead to erroneous calibration, ultimately affecting the final test results. To account for noncommutability, a maximum allowable noncommutability bias (umaxNC) is determined and incorporated into the overall uncertainty budget.
Establishing APS for Commutability
To mitigate the effects of noncommutability, ISO standards and guidelines provide methods for setting an APS for umaxNC. This involves assessing the bias between clinical samples and RMs from different IVD-MDs and ensuring that the bias does not exceed the maximum allowable limits. Commutability assessments are crucial for maintaining the integrity of the calibration process and ensuring that test results remain consistent across laboratories and diagnostic systems.
Addressing Variability Across Different IVD-MDs and Laboratories
Inter-Laboratory Variability

Even with strict metrological traceability and APS, variability may still exist between different laboratories due to differences in IVD-MDs, reagent lots, and operational conditions. This inter-laboratory variability can introduce additional uncertainty that is not captured by the umaxCS or uRw alone.
To address this, a more holistic approach is needed that considers total allowable error, which includes not only the uncertainty at each calibration step but also the potential biases and errors from inter-laboratory variability, reagent lot changes, and pre-analytical factors.
Total Allowable Error in Clinical Laboratories

Total allowable error takes into account all sources of uncertainty and bias, including those that arise from the operation of multiple IVD-MDs, reagent lot variations, and inter-laboratory differences. This broader definition of error provides a more realistic estimate of the uncertainty associated with clinical test results, ensuring that the final result meets the clinical requirements for accuracy and reliability.
Conclusion: Enhancing Diagnostic Accuracy Through Metrological Traceability
Metrological traceability and analytical performance specifications are critical to ensuring that clinical diagnostic results are both accurate and consistent. However, challenges such as repeatability uncertainty, noncommutability of reference materials, and inter-laboratory variability must be carefully managed to maintain the integrity of the measurement process.
As laboratories continue to adopt advanced IVD-MDs and improve their operational processes, it will be essential to refine the methods used to estimate uncertainty, ensuring that all sources of error are captured and controlled. By doing so, laboratories can continue to provide high-quality diagnostic services that support informed medical decision-making and ultimately improve patient outcomes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- Greg Miller, W. "The role of analytical performance specifications in international guidelines and standards dealing with metrological traceability in laboratory medicine." Clinical Chemistry and Laboratory Medicine (CCLM) 62.8 (2024): 1462-1469.
This article is for research use only. Do not use in any diagnostic or therapeutic application.
Trending Products



 Fig.1 Metrological traceability as defined in ISO 17511:2020. (Greg M. W., et al., 2024)
Fig.1 Metrological traceability as defined in ISO 17511:2020. (Greg M. W., et al., 2024) 


































