- Home
- Resource
- Disease Diagnosis
- Metabolic Diseases
- The Diagnostic Pathway for Propionic Acidemia (PA): Biomarkers, Enzymatics, and Genetics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
The accurate diagnosis of propionic acidemia (PA) requires a coordinated multi-method approach that integrates biochemical, enzymatic, and genetic analyses. This resource details the complete diagnostic pathway, from newborn screening using tandem mass spectrometry through definitive genetic confirmation, providing clinicians and laboratory professionals with a systematic framework for timely and accurate diagnosis.
Propionic acidemia (PA) is a rare, life-threatening autosomal recessive disorder caused by a deficiency in the enzyme propionyl-CoA carboxylase, which is essential for metabolizing specific amino acids and fatty acids. This defect leads to the toxic accumulation of organic acids like propionic acid and its derivatives in the body, resulting in severe metabolic crises characterized by poor feeding, vomiting, lethargy, and hyperammonemia. If untreated, the condition can progress to profound neurological impairment or death, making rapid diagnosis through biomarker screening, enzymatic testing, and genetic confirmation critical for initiating life-saving dietary management and medical treatment.
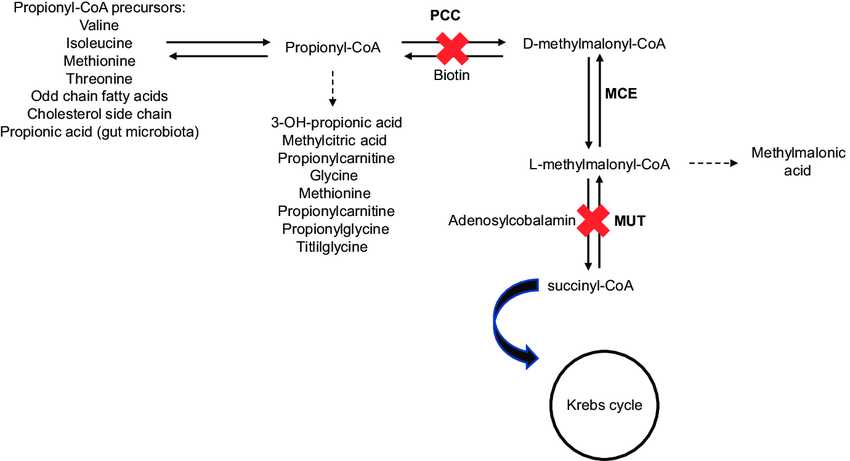 Fig.1 Metabolic pathway of propionate. (Medina-Torres, E. A., et al., 2021)
Fig.1 Metabolic pathway of propionate. (Medina-Torres, E. A., et al., 2021)
The diagnosis of propionic acidemia (PA) relies heavily on the detection of specific biochemical biomarkers that accumulate due to the functional deficiency of propionyl-CoA carboxylase. These biomarkers provide crucial evidence at different stages of the diagnostic pathway, from initial newborn screening to definitive biochemical confirmation.

Newborn Screening-Propionylcarnitine (C3)
The initial and crucial indicator for propionic acidemia (PA) in newborn screening is the significant elevation of propionylcarnitine (C3) detected through tandem mass spectrometry (MS/MS) analysis of dried blood spots. An elevated C3 level, particularly when combined with an increased C3/C2 (acetylcarnitine) ratio, provides a highly sensitive screen that triggers the urgent need for definitive diagnostic testing.
Diagnostic Confirmation-Urinary Organic Acids
Definitive biochemical confirmation of PA relies on gas chromatography-mass spectrometry (GC-MS) analysis of urinary organic acids, which reveals a characteristic and diagnostic pattern of elevated metabolites:

Enzymatic assays provide definitive functional confirmation of propionic acidemia (PA) by directly measuring the activity of propionyl-CoA carboxylase (PCC) in cultured patient cells, such as fibroblasts or lymphocytes. This test is crucial because it moves beyond identifying accumulating biomarkers to directly demonstrating the underlying enzymatic deficiency, thereby conclusively validating the biochemical diagnosis. A confirmed diagnosis enables accurate genetic counseling and is particularly valuable for guiding family studies and prenatal diagnosis, especially in cases where genetic test results are ambiguous or variants of unknown significance are identified.
Genetic analysis represents the definitive level of diagnosis for propionic acidemia (PA), moving from the biochemical consequences of the disease to identifying its root genetic cause. This involves testing for pathogenic variants in two specific genes, PCCA and PCCB, which encode the alpha and beta subunits, respectively, of the propionyl-CoA carboxylase (PCC) enzyme. Biallelic pathogenic mutations in either of these genes disrupt the assembly or function of the PCC enzyme complex, leading to the disease.
The modern diagnosis of propionic acidemia (PA) follows a streamlined, multi-tiered pathway that integrates complementary laboratory methods to ensure both speed and accuracy. This approach systematically progresses from initial screening to biochemical confirmation and finally to definitive molecular characterization, with each step validating and refining the previous one.

The diagnostic pathway initiates with newborn screening using tandem mass spectrometry (MS/MS) to analyze dried blood spots. This first-line screening detects elevated levels of propionylcarnitine (C3) and an increased C3/C2 ratio, serving as crucial early indicators that trigger immediate further investigation for at-risk infants before symptom onset.

Following a positive screening result, definitive biochemical confirmation is obtained through urinary organic acid analysis via gas chromatography-mass spectrometry (GC-MS). This essential step identifies the characteristic metabolic pattern of markedly elevated compounds including methylcitrate, 3-hydroxypropionate, and propionylglycine, providing conclusive evidence of the metabolic derangement.
The diagnostic pathway culminates with definitive testing through either enzymatic or genetic analysis. Enzymatic assays measure propionyl-CoA carboxylase activity to functionally confirm the deficiency, while genetic testing identifies biallelic pathogenic variants in the PCCA or PCCB genes, providing molecular confirmation and enabling family studies.
Advancing beyond traditional testing approaches, Alta DiagnoTech's diagnostic solutions enable a seamless transition from initial screening to comprehensive confirmation for propionic acidemia (PA). This integrated portfolio supports the complete diagnostic pathway, providing laboratories with the precision tools needed to deliver timely and definitive results throughout the clinical decision-making process. If you have related needs, please feel free to contact us for more information or product support.
| Product Category | Product Name | Description |
| Newborn Screening | C3 Acylcarnitine Quantitative Assay Kit (DBS) | First-line newborn screening for PA; MS/MS-based quantification of propionylcarnitine (C3) with C3/C2 ratio calculation. |
| Biochemical Confirmation | PA Urinary Metabolite Panel (GC-MS) | Definitive biochemical confirmation; multaneous detection of methylcitrate, 3-hydroxypropionate, and propionylglycine. |
| Enzymatic Confirmation | Propionyl-CoA Carboxylase (PCC) Activity Assay | Functional confirmation of diagnosis; Measures PCC enzyme activity in cultured fibroblasts. |
| Genetic Confirmation | PA Genetic Analysis Panel | Molecular diagnosis and carrier testing; Comprehensive sequencing of PCCA and PCCB genes. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |