- Home
- Resource
- Explore & Learn
- The Complex World of Diagnostic Regulation and Innovation
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Diagnostic tools form the cornerstone of effective healthcare, guiding clinical decisions at the patient's bedside and shaping public health strategies on a global scale. They are indispensable in identifying infections, monitoring treatment responses, and preventing the spread of contagious diseases. Yet, despite their importance, a significant portion of the global population remains underserved, with limited access to reliable, affordable testing. This gap is particularly pronounced in low- and middle-income countries (LMICs), where healthcare infrastructure is often inadequate and regulatory frameworks for medical devices are underdeveloped or nonexistent.
The consequences of this disparity are far-reaching. Without timely and accurate diagnostics, diseases such as tuberculosis, malaria, and drug-resistant infections go undetected, leading to prolonged illness, increased transmission, and avoidable deaths. For instance, in sub-Saharan Africa, where tuberculosis remains a leading cause of morbidity, the inability to rapidly diagnose drug-resistant strains means patients often receive ineffective treatments, exacerbating antimicrobial resistance (AMR) and straining already overburdened healthcare systems.
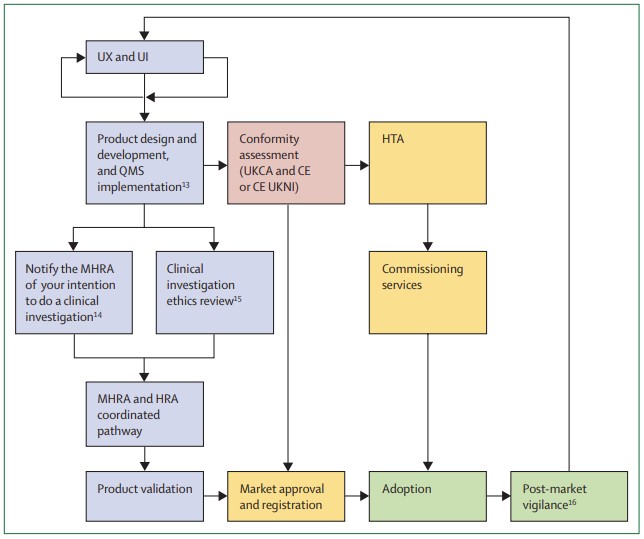 Fig.1 Diagnostic product journey in the UK. (Rodriguez-Manzano J., et al., 2024)
Fig.1 Diagnostic product journey in the UK. (Rodriguez-Manzano J., et al., 2024)
In high-income countries, while access to diagnostics is generally better, challenges persist. Innovators developing cutting-edge technologies—such as AI-powered molecular tests or digital diagnostics—face a labyrinth of regulatory requirements that vary by region, slowing down the translation of promising innovations into clinical practice. The COVID-19 pandemic highlighted both the critical need for rapid diagnostic deployment and the systemic bottlenecks that can hinder timely access, even in resource-rich settings.

The regulatory environment for in vitro diagnostics (IVDs) is characterized by complexity and fragmentation. Unlike pharmaceuticals, which have relatively standardized global regulatory pathways, diagnostics are governed by a mosaic of rules that differ significantly between countries and regions. This is partly due to the diverse nature of diagnostic technologies—ranging from simple lateral flow tests to sophisticated genomic assays—and their varying risk profiles, which make one-size-fits-all regulation impractical.
In the European Union, the introduction of Regulations 2017/745 and 2017/746 aimed to harmonize the regulatory framework for medical devices and IVDs. However, implementation has been fraught with delays, particularly for high-risk IVDs. Manufacturers often face wait times of up to 18 months for review by notified bodies—accredited organizations responsible for conformity assessments—creating uncertainty and slowing market entry. Similarly, in the United States, while the FDA's Breakthrough Devices Program has expedited access to innovative technologies, the pre-submission process and post-market surveillance requirements remain rigorous, requiring significant investment of time and resources.
For LMICs, the regulatory challenges are even more acute. Many countries lack functional national regulatory authorities (NRAs) capable of evaluating and approving IVDs. Those that do exist often struggle with limited staffing, inadequate funding, and a shortage of technical expertise. As a result, these countries frequently rely on regulatory approvals from other jurisdictions, such as the EU or the US, but this dependence can create delays and limit access to technologies tailored to local needs. The World Health Organization's (WHO) prequalification program has helped address this gap by evaluating IVDs for use in LMICs, but it currently covers only 15 types of medical devices, leaving many critical diagnostics unassessed.
In response to these challenges, several initiatives have emerged to streamline regulatory processes and accelerate access to innovative diagnostics. In the United Kingdom, the Medicines and Healthcare products Regulatory Authority (MHRA) launched the Innovative Devices Access Pathway (IDAP) in 2024. This program aims to simplify approval by providing early guidance to innovators, coordinating reviews across different regulatory bodies, and facilitating knowledge exchange between developers and approved certification bodies. By aligning with international efforts such as the FDA's Breakthrough Devices Program, IDAP seeks to reduce duplication and create a more predictable pathway for market entry.

Internationally, the International Medical Device Regulators Forum (IMDRF) has played a key role in promoting harmonization. Building on the work of its predecessor, the Global Harmonization Task Force (GHTF), IMDRF develops guidance documents that align regulatory practices across member countries, from risk classification to post-market surveillance. This collaboration has been particularly valuable for emerging technologies, such as software-based diagnostics and AI-driven tools, where innovation often outpaces regulation.
Regional initiatives have also made significant strides. In Africa, the African Medical Devices Forum (AMDF), part of the African Medicines Regulatory Harmonization program, has worked to operationalize IMDRF principles across the continent. During the COVID-19 pandemic, AMDF facilitated regional cooperation on emergency use authorizations for diagnostics, demonstrating the potential of harmonized approaches to address public health crises. More recently, it has extended this model to address outbreaks such as mpox, coordinating expedited evaluations across affected countries to ensure rapid access to reliable tests.

Despite these advances, the diagnostic gap between high-income countries and LMICs remains stark. In many parts of Africa, Asia, and Latin America, even basic diagnostics are scarce. For example, rapid diagnostic tests for malaria—while widely available in urban centers—often fail to reach rural communities, where the disease burden is highest. This is due in part to regulatory barriers, but also to infrastructure limitations, including unreliable electricity, poor transportation networks, and inadequate laboratory facilities.
Digital diagnostics offer promise for bridging this gap. These technologies combine molecular detection capabilities with point-of-care convenience and mobile connectivity, enabling real-time data transmission and remote monitoring. For instance, digital PCR devices can now detect malaria parasites with high sensitivity in resource-constrained settings, while smartphone apps can analyze test results and share data with central health authorities. However, their adoption in LMICs is hindered by several factors: unclear regulatory guidelines for software-based tools, concerns about data privacy and security, and the need for robust validation in diverse clinical contexts.
To overcome these hurdles, a multifaceted approach is required. Strengthening local regulatory capacity is critical—this includes training staff, developing sustainable funding mechanisms, and implementing WHO's Global Model Regulatory Framework, which provides a roadmap for aligning national regulations with international standards. Additionally, initiatives such as the Foundation for Innovative New Diagnostics (FIND)'s partnership with Unitaid are working to address bottlenecks in manufacturing and supply chains, supporting local production of IVDs and reducing reliance on imported technologies.
Looking forward, the future of diagnostic regulation will depend on striking a balance between fostering innovation and ensuring safety. One promising development is the shift toward "pull" models of innovation, where clinical needs drive development rather than technological capabilities alone. Target Product Profiles (TPPs)—detailed specifications outlining the desired characteristics of a diagnostic—have proven effective in this regard. During the COVID-19 pandemic, TPPs helped focus developer efforts on tests that met specific criteria, from sensitivity thresholds to cost targets, resulting in more practical and widely adoptable technologies.

Another key area is post-market surveillance. Regulators are increasingly recognizing that rigorous monitoring after approval can complement pre-market evaluations, allowing for faster initial authorization while ensuring ongoing safety. For example, the MHRA's YellowCard scheme enables healthcare professionals and the public to report adverse events involving medical devices, providing valuable real-world data on performance. Similarly, the FDA's post-market requirements for manufacturers ensure that any issues with diagnostics are addressed promptly, from labeling updates to product recalls.
Finally, collaboration across stakeholders—including regulators, innovators, clinicians, and patient groups—will be essential. The UK's National Action Plan on AMR (2024–2029) exemplifies this approach, prioritizing diagnostics as a tool to combat drug resistance and aligning regulatory frameworks with international standards to ensure consistency. By bringing together diverse perspectives, such initiatives can create regulatory environments that support innovation while addressing global health priorities.
The landscape of diagnostic regulation is complex, but progress is being made. From streamlined pathways in high-income countries to regional harmonization efforts in LMICs, there is growing recognition that effective regulation must balance safety, innovation, and equity. As diagnostic technologies continue to evolve—from microbiome-based tests to AI-driven platforms—the need for flexible, collaborative regulatory approaches will only increase.
Ultimately, the goal is clear: to ensure that reliable, affordable diagnostics are accessible to all, regardless of geography or socioeconomic status. Achieving this will require ongoing commitment to harmonization, capacity building, and patient-centered innovation. By navigating the regulatory maze together, we can unlock the full potential of diagnostics to transform healthcare and address global challenges such as antimicrobial resistance.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |