- Home
- Resource
- Explore & Learn
- The Complex World of Companion Diagnostics: Challenges and the Path to Innovation
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Companion diagnostics (CDx) have emerged as an indispensable component of precision medicine, providing critical information to guide the safe and effective use of targeted therapies. These diagnostic tests are specifically designed to identify patients who are most likely to benefit from a particular therapeutic intervention, thereby optimizing treatment outcomes and minimizing adverse effects. However, the current regulatory landscape and the rapidly evolving nature of biomarker research have introduced significant complexities that challenge the innovation and implementation of companion diagnostics. This article explores the multifaceted challenges faced by the CDx industry and proposes potential solutions to foster innovation while ensuring patient safety and accessibility.
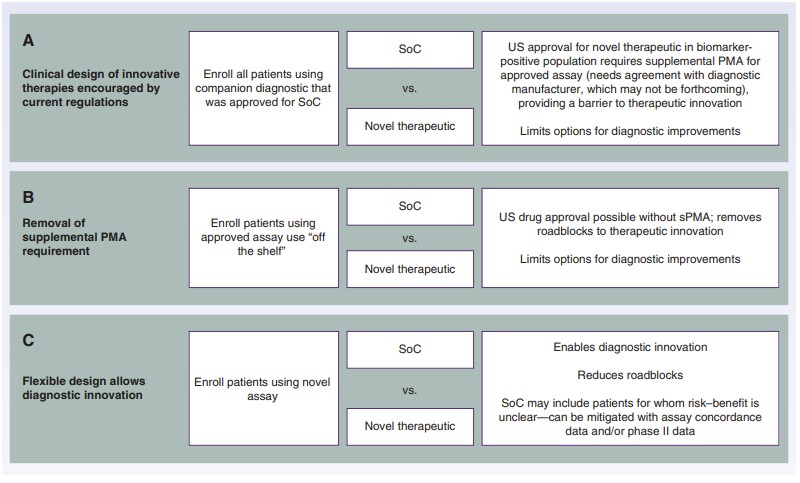 Fig.1 Clinical trial design options in biomarker-positive populations defined by a companion diagnostic test. (Oliner K. S., et al., 2025)
Fig.1 Clinical trial design options in biomarker-positive populations defined by a companion diagnostic test. (Oliner K. S., et al., 2025)

The development and approval of companion diagnostics are governed by stringent regulatory frameworks aimed at ensuring the analytical and clinical validity of these tests. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan require robust evidence of a test's performance before granting marketing authorization. While these regulations have enhanced the reliability and consistency of biomarker testing, they have also inadvertently created barriers to innovation. The one-drug/one-test model, for instance, has led to a fragmented diagnostic landscape, where multiple assays are developed for the same biomarker, each with varying cut-offs and scoring algorithms. This complexity not only confuses clinicians and pathologists but also increases the burden on laboratories and healthcare systems.
One of the most pressing issues in the companion diagnostics field is the approval of multiple assays for the same biomarker. For example, in the realm of PD-L1 testing for immunotherapies, four different assays have been approved, each with distinct expression cut-offs and scoring algorithms. This multiplicity of tests creates confusion and inconsistency in clinical practice. Clinicians may struggle to determine which assay to order, while pathologists face challenges in interpreting and validating multiple tests with varying criteria. Moreover, the need for laboratories to offer multiple assays for the same biomarker is resource-intensive and financially burdensome. In a 2022 survey, only three of the top 20 U.S. clinical laboratories for non-small cell lung cancer (NSCLC) testing were able to offer all four commercially available PD-L1 companion diagnostic tests.

The terminology used in drug labeling further complicates the adoption of companion diagnostics. The term "approved assay" implies that only the specific assay approved by regulators can be used, limiting the flexibility to adopt newer, potentially better-performing assays. For instance, the approval of erdafitinib for metastatic bladder cancer highlights the confusion caused by varying genetic alterations detected by different assays. The approved companion diagnostic test detects specific FGFR2/3 mutations and translocations, but the drug's prescribing information mentions additional fusions, leading to inconsistent interpretation of susceptible genetic alterations. This ambiguity can hinder the widespread adoption of innovative diagnostics and limit patient access to optimal treatments.
Current regulations require that any new therapeutic in a biomarker-selected population must be tested using the approved companion diagnostic. This requirement can impede the development of innovative diagnostics and therapeutics, as it limits the ability to explore new assays or algorithms that may better identify responding patients. Additionally, the need for a supplemental pre-market authorization (PMA) for new diagnostics can create roadblocks if diagnostic companies refuse to partner with pharmaceutical firms. This scenario is particularly problematic when diagnostic companies have exclusive commercial relationships or insufficient production capacity to meet market demands.

The current regulatory environment has several unintended consequences that impact innovation and accessibility:
To address these challenges, several practical solutions have been proposed:
Concordance studies, which compare the results of different assays, can help laboratories understand whether multiple tests are necessary or if a single test can suffice. For example, cross-party harmonization initiatives for PD-L1 assays have shown good concordance in non-small cell lung cancer (NSCLC), although results vary in other cancer types. These studies emphasize the importance of correct sample handling and processing in diagnostic testing.
Regulators could consider using terminology like "analytically validated test" instead of "approved test" in drug labels. This change would allow broader access to testing while ensuring quality. Additionally, regulators could approve companion diagnostics for a drug class rather than individual drugs, simplifying the process.
Pharmaceutical companies could be encouraged to generate efficacy data using multiple diagnostic assays in pivotal clinical trials. This approach would provide valuable information on the comparative performance of different assays and support the development of new diagnostics. Quality of testing could be assured by using the approved assay in laboratories with ISO 15189 accreditation or College of American Pathologists (CAP)/Clinical Laboratory Improvement Amendments (CLIA) certification.
Regulators could permit the use of new diagnostics in phase III studies, even for the standard-of-care arm, provided that the risk-benefit profile is established through phase I/II data. This flexibility would allow innovative assays to be tested in real-world settings without compromising patient safety. Additionally, the FDA's Oncology Drug Products Used with Certain In Vitro Diagnostics Pilot Program could be expanded to include more indications and populations.
The field of companion diagnostics holds immense promise for advancing precision medicine. However, the current regulatory landscape presents significant challenges that could stifle innovation and limit patient access to potentially life-saving treatments. By adopting flexible regulatory approaches, promoting assay concordance studies, and encouraging the generation of comparative efficacy data, we can create an environment that fosters innovation while maintaining the highest standards of patient care. Collaboration between regulators, pharmaceutical companies, molecular pathologists, and diagnostic developers is essential to navigate these complexities and ensure that precision medicine reaches its full potential.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |