- Home
- Resource
- Disease Diagnosis
- Endocrine Diseases
- The Biochemical and Imaging Cornerstones of Acromegaly and Gigantism Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Acromegaly and gigantism are clinical disorders resulting from chronic excess of growth hormone (GH), leading to progressive somatic changes and systemic complications. This resource details the established diagnostic pathway for these conditions, beginning with the critical role of biochemical confirmation using key biomarkers like IGF-1 and dynamic testing. It further explains the pivotal step of imaging localization to identify the causative lesion, most commonly a pituitary adenoma, and concludes with an outlook on future diagnostic trends. An overview of the specialized in vitro diagnostic (IVD) tools that support this entire process is also provided.
Acromegaly and gigantism are two clinical manifestations of the same underlying disorder: chronic, excessive secretion of growth hormone (GH), most commonly caused by a benign pituitary adenoma. The distinction is based on the timing of onset relative to skeletal maturation. Gigantism results from GH excess occurring in childhood or adolescence before the closure of the epiphyseal growth plates, leading to excessive linear growth and tall stature. Acromegaly occurs in adulthood after growth plates have fused, characterized by the gradual, disproportionate enlargement of bones and soft tissues in the hands, feet, and face. Both conditions are associated with significant systemic complications, including cardiovascular disease, diabetes, arthritis, and reduced life expectancy if left untreated. Diagnosis is often delayed due to the insidious onset of symptoms, making a high index of clinical suspicion and systematic biochemical evaluation critical.
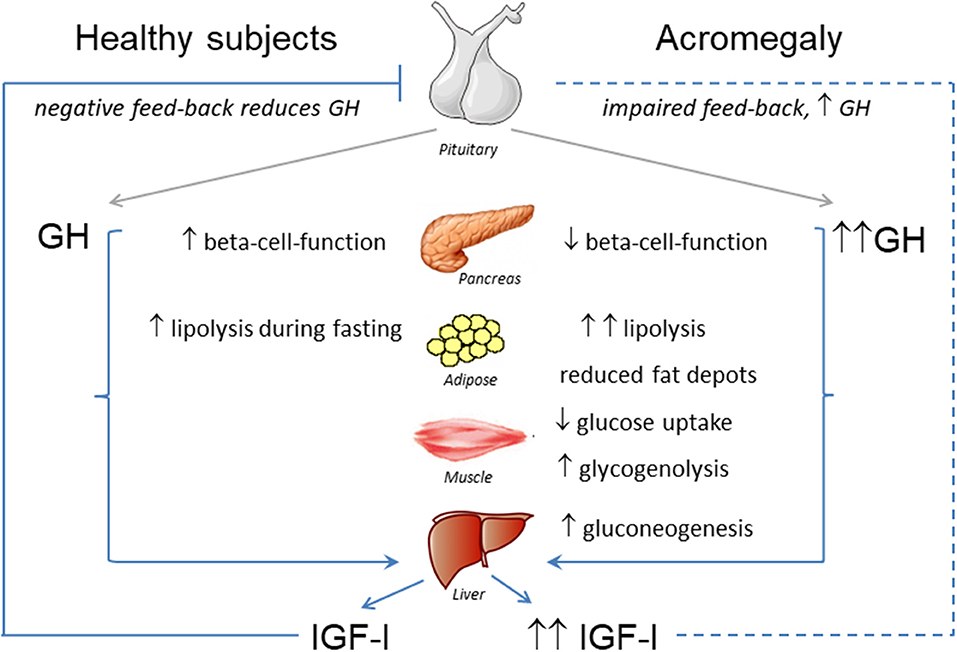 Fig.1 Effects of GH-IGF-I hypersecretion in metabolic organs. (Vila G, et al., 2019)
Fig.1 Effects of GH-IGF-I hypersecretion in metabolic organs. (Vila G, et al., 2019)
Confirming the diagnosis of acromegaly and gigantism relies on a sequence of specialized biochemical tests designed to objectively demonstrate chronic growth hormone (GH) excess. Due to the pulsatile nature of GH secretion, random GH measurements are unreliable for diagnosis. Therefore, a structured biochemical approach is essential, beginning with a sensitive screening biomarker, followed by a dynamic confirmatory test, and supplemented by additional hormonal assessments to provide a comprehensive clinical picture.
Screening Biomarker – Insulin-like Growth Factor 1 (IGF-1)
Serum insulin-like growth factor 1 (IGF-1) serves as the cornerstone screening test. As the primary mediator of GH action, IGF-1 provides a stable, integrated reflection of GH secretion over time, smoothing out its pulsatility. An elevated age-adjusted serum IGF-1 level is highly sensitive for detecting GH excess. Accurate interpretation requires comparison to method-specific and age-stratified reference ranges.

Gold-Standard Confirmatory Test – Oral Glucose Tolerance Test (OGTT)
The oral glucose tolerance test (OGTT) is the definitive confirmatory procedure. In healthy individuals, a glucose load normally suppresses pituitary GH secretion. In acromegaly, this feedback is disrupted. The diagnostic criterion is the failure of GH to suppress below 1 µg/L (or below 0.4 µg/L with ultrasensitive assays) at any point during the 75g OGTT. This test provides direct proof of autonomous GH secretion.
Additional Biochemical Tools
While IGF-1 and the OGTT form the diagnostic core, additional tests provide valuable supporting information. Measurement of other pituitary hormones (e.g., prolactin, TSH, ACTH) is crucial to identify commonly co-secreting adenomas and assess overall pituitary function. In cases of suspected ectopic GHRH secretion, plasma GHRH measurement may be indicated. These tools aid in characterizing the tumor and guiding comprehensive management.
Once biochemical tests confirm growth hormone (GH) excess, precise anatomical localization of the source is the critical next step to guide definitive therapy. Imaging aims to identify and characterize the causative lesion, which is most commonly a pituitary adenoma, and to assess its relationship to critical surrounding structures. The choice and sequence of imaging modalities are tailored to provide the detailed anatomical and, when necessary, functional information required for treatment planning.
Pituitary MRI with gadolinium contrast is the cornerstone of anatomical localization, providing the high-resolution detail necessary to detect and characterize a pituitary adenoma. It precisely defines the lesion's size, location, and relationship to critical structures like the cavernous sinus and optic chiasm. This information is essential for determining surgical feasibility and approach.
When standard MRI fails to identify a clear source despite biochemical confirmation, an ectopic source of GH or GHRH must be investigated. Functional imaging with somatostatin receptor PET/CT is the key next step for detecting neuroendocrine tumors. Anatomical surveys of the chest and abdomen via CT may also be performed, while a sellar CT offers an alternative only if MRI is contraindicated.

The future of acromegaly and gigantism diagnostics is moving towards greater precision, earlier detection, and a more holistic disease assessment. This evolution is being driven by advancements in ultrasensitive and multiplexed biomarker assays, such as mass spectrometry-based hormone panels and novel GH isoforms, which promise improved diagnostic accuracy and earlier biochemical detection. Integration of artificial intelligence for image analysis will enhance the interpretation of pituitary MRI and functional imaging. Furthermore, the growing application of liquid biopsy techniques to analyze tumor-derived biomarkers in blood may eventually allow for non-invasive tumor genotyping and real-time monitoring of treatment response, paving the way for truly personalized management strategies.
With cutting-edge technology and a dedicated team, Alta DiagnoTech offers a comprehensive portfolio of in vitro diagnostic (IVD) products for acromegaly and gigantism, supporting the entire diagnostic process from initial suspicion to treatment monitoring. Our solutions are designed to address the specific challenges of diagnosing hyperglycemia, providing accurate and reliable detection methods for key biomarkers and supporting advanced diagnostic technologies. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Insulin-like Growth Factor 1 (IGF-1) CLIA Kit | Automated Chemiluminescent Immunoassay (CLIA) | Quantitative measurement of serum IGF-1 as the primary screening biomarker for chronic GH excess. Provides age-adjusted reference ranges for accurate interpretation. |
| Growth Hormone (GH) CLIA Kit | Automated Chemiluminescent Immunoassay (CLIA) | Measurement of serum GH levels, primarily used in conjunction with the Oral Glucose Tolerance Test (OGTT) for definitive diagnosis and disease activity monitoring. |
| Ultrasensitive Growth Hormone (US-GH) CLIA Kit | High-Sensitivity Chemiluminescent Immunoassay (CLIA) | Enables precise quantification of very low GH concentrations (<0.05 µg/L), critical for accurately interpreting the OGTT using modern stringent criteria (GH nadir <0.4 µg/L). |
| Growth Hormone Releasing Hormone (GHRH) ELISA Kit | Enzyme-Linked Immunosorbent Assay (ELISA) | Measurement of plasma GHRH levels to aid in the differential diagnosis of ectopic GHRH secretion in cases where a pituitary adenoma is not visualized. |
| Pituitary Hormone Panel CLIA Kits (Prolactin, TSH, FSH, LH, ACTH) | Automated Chemiluminescent Immunoassay (CLIA) | Comprehensive assessment of other pituitary axes to evaluate for co-secreting adenomas and assess overall pituitary function prior to intervention. |
| Multiplexed Pituitary Hormone Mass Spectrometry Panel | Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) | Simultaneous, high-specificity quantification of GH, IGF-1, and other relevant pituitary hormones from a single sample. Ideal for complex cases and research-oriented precision diagnostics. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |