CRISPR/Cas systems have revolutionized molecular diagnostics, providing exceptional precision and flexibility for nucleic acid detection. While traditional diagnostic methods like quantitative polymerase chain reaction (qPCR) and next-generation sequencing (NGS) have long been considered the gold standards in clinical practice, they often face limitations due to their complexity, high costs, and the requirement for specialized equipment and skilled personnel. In contrast, CRISPR/Cas-based diagnostics (CRISPRDx) overcome these challenges by harnessing the programmable capabilities of CRISPR systems, thereby enabling rapid, accurate, and cost-effective detection of pathogens, genetic mutations, and other biomarkers.
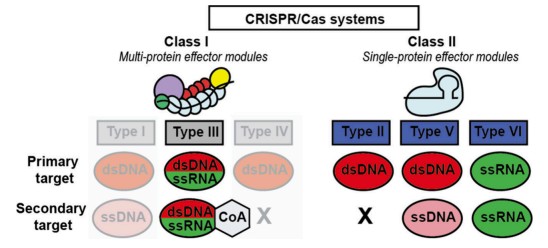 Fig.1 Classification of CRISPR/Cas systems is based on two classes defined by their crRNA−effector protein complex. (van Dongen J. E., et al., 2025)
Fig.1 Classification of CRISPR/Cas systems is based on two classes defined by their crRNA−effector protein complex. (van Dongen J. E., et al., 2025)
The Mechanism of CRISPR/Cas Systems
CRISPR/Cas systems are divided into two main categories based on their effector proteins and mechanisms of action. Class I systems, exemplified by type III-A, rely on multiprotein complexes to identify and cleave target nucleic acids. These systems generate signaling molecules, such as cyclic oligonucleotides (CoAs) and protons (H+), which can be detected using colorimetric or fluorometric approaches. In contrast, Class II systems, which encompass Cas9, Cas12, and Cas13, are favored for in vitro diagnostics due to their streamlined, single-protein effector components. These systems not only perform targeted cleavage but also exhibit collateral cleavage activity, which markedly improves detection sensitivity.
| CRISPR System |
Mechanism |
Application |
| Class I (Type III-A) |
Multiprotein complex, produces CoAs and H+ |
Colorimetric and fluorometric detection |
| Class II (Cas9, Cas12, Cas13) |
Single-protein effector, collateral cleavage |
Fluorescent and colorimetric detection |
CRISPRDx in Rapid Point-of-Care Testing
A particularly promising area for CRISPR-based diagnostic tools (CRISPRDx) is in the development of rapid point-of-care (PoC) testing. During the COVID-19 pandemic, platforms such as SHERLOCK and DETECTR highlighted the potential of CRISPRDx for swift and precise detection of the SARS-CoV-2 virus. These innovative platforms capitalize on the collateral cleavage activity of Cas enzymes to generate fluorescent signals when a target is detected, enabling high-throughput testing in just 1−2 hours. For instance, the SHERLOCK platform utilizes Cas13 to identify viral RNA, whereas DETECTR relies on Cas12 for DNA-based detection. These methods achieve accuracy comparable to traditional PCR tests, but with dramatically reduced turnaround times.
Detection of Low-Abundant Mutations and SNPs
CRISPRDx demonstrates remarkable proficiency in detecting low-abundance mutations and single-nucleotide polymorphisms (SNPs), which are frequently overlooked by conventional PCR techniques. For example, Cas12a is capable of detecting SNPs at abundance levels as low as 0.5%, especially when used in conjunction with PCR. Predictive tools such as ARTEMIS aid in pinpointing targetable SNPs within PAM-proximal seed regions, thereby enhancing the specificity of CRISPR-based detection methods. This capability is particularly vital in cancer diagnostics, where identifying rare mutations can inform the selection of targeted therapies.
Monitoring Gene Expression and Epigenetic Modifications
In addition to detecting pathogens and mutations, CRISPRDx is now being applied to monitor gene expression profiles and identify epigenetic modifications. These capabilities are especially significant in cancer diagnostics, as detecting rare mutations and tracking epigenetic alterations can offer crucial information for personalized treatment strategies. For instance, CRISPR/Cas12a-based systems are capable of quantifying single CpG methylation sites, providing a robust means for early cancer detection and ongoing monitoring.
Technical and Logistical Challenges
While CRISPRDx holds great promise, it currently faces several technical and logistical hurdles. One major issue is the detection limit, as the enzyme kinetics of existing CRISPR systems restrict sensitivity to picomolar (pM) levels without preamplification. To meet the demands of applications such as liquid biopsy diagnostics, achieving amplification-free, subfemtomolar detection sensitivity is critical. This will likely require innovations like improving the trans-cleavage efficiency of CRISPR proteins.
Furthermore, CRISPRDx assays often necessitate sample processing steps, such as lysis or pretreatment, which add complexity. Additionally, the requirement for ultralow temperature storage poses significant challenges for deployment in resource-limited settings. Overcoming these obstacles is essential for transforming CRISPRDx from laboratory prototypes into fully deployable point-of-care (PoC) solutions.
Regulatory and Ethical Considerations
For CRISPRDx to achieve widespread implementation, regulatory approval and ethical considerations are of paramount importance. In the United States, CRISPRDx technologies must adhere to the regulatory frameworks established by the FDA for in vitro diagnostics (IVDs). High-risk disease detection applications typically necessitate more stringent premarket approval (PMA) processes. Similarly, in the European Union, CRISPRDx is governed by the In Vitro Diagnostic Regulation (IVDR), which enforces comprehensive regulatory oversight, especially for high-risk diagnostic tools.
Ethical issues related to privacy and data security also come to the forefront, given that CRISPRDx generates highly sensitive genetic information that is susceptible to misuse. Developers must prioritize stringent data protection measures and ensure compliance with relevant regulations, such as the General Data Protection Regulation (GDPR) in the European Union and the Health Insurance Portability and Accountability Act (HIPAA) in the United States.
Future Perspectives and Research Directions
The future trajectory of CRISPR/Cas diagnostics hinges on several key advancements. These include improving detection sensitivity without the need for preamplification, ensuring seamless integration into clinical workflows, and developing rapid, single-step assays that can be easily adapted to existing diagnostic infrastructure. Promising innovations in this direction involve enhancing the trans-cleavage efficiency of CRISPR proteins and leveraging microfluidics to create digital CRISPR sensors, both of which have the potential to overcome current limitations.
For point-of-care (PoC) applications, CRISPRDx must meet the World Health Organization's ASSURED criteria, which emphasize affordability, robustness, and minimal infrastructure requirements. Ongoing research and development efforts in these areas will be essential for unlocking the full potential of CRISPRDx in clinical diagnostics and ensuring its widespread adoption.
Conclusion
CRISPR/Cas diagnostics have emerged as a groundbreaking development in the field of molecular diagnostics, providing swift, precise, and economically viable methods for identifying pathogens, genetic mutations, and other critical biomarkers. Despite existing challenges related to detection sensitivity, technical intricacy, and regulatory requirements, ongoing advancements and refinements in CRISPRDx are poised to revolutionize clinical diagnostics and enhance patient care. As researchers and industry stakeholders collaborate to surmount these obstacles, CRISPR technology is set to become a fundamental component of next-generation diagnostic approaches, fostering innovation and elevating diagnostic standards across both clinical and point-of-care settings.
If you have related needs, please feel free to contact us for more information or product support.
Reference
- van Dongen, Jeanne E., and Loes I. Segerink. "Building the Future of Clinical Diagnostics: An Analysis of Potential Benefits and Current Barriers in CRISPR/Cas Diagnostics." ACS Synthetic Biology (2025).
This article is for research use only. Do not use in any diagnostic or therapeutic application.
Trending Products



 Fig.1 Classification of CRISPR/Cas systems is based on two classes defined by their crRNA−effector protein complex. (van Dongen J. E., et al., 2025)
Fig.1 Classification of CRISPR/Cas systems is based on two classes defined by their crRNA−effector protein complex. (van Dongen J. E., et al., 2025)