- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- SARS Diagnostics Uncovered: Advanced Methods, Biomarkers, and Emerging Technologies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Severe acute respiratory syndrome (SARS) remains a pivotal case in epidemic response, highlighting the need for accurate and rapid diagnosis. This resource provides a comprehensive overview of SARS detection methods, including RT-PCR, serological testing, and antigen detection. It also describes biomarkers and cutting-edge innovative technologies for SARS diagnosis.
Severe acute respiratory syndrome (SARS) is a viral respiratory illness caused by SARS-associated coronavirus (SARS-CoV), first identified in 2002-2003. Characterized by high fever, dry cough, and progressive pneumonia, SARS spreads via respiratory droplets and has a high fatality rate (~10%). While no cases have been reported since 2004, SARS remains a prototype for pandemic preparedness, highlighting the need for rapid diagnostics to distinguish it from other coronaviruses (e.g., SARS-CoV-2, MERS-CoV).
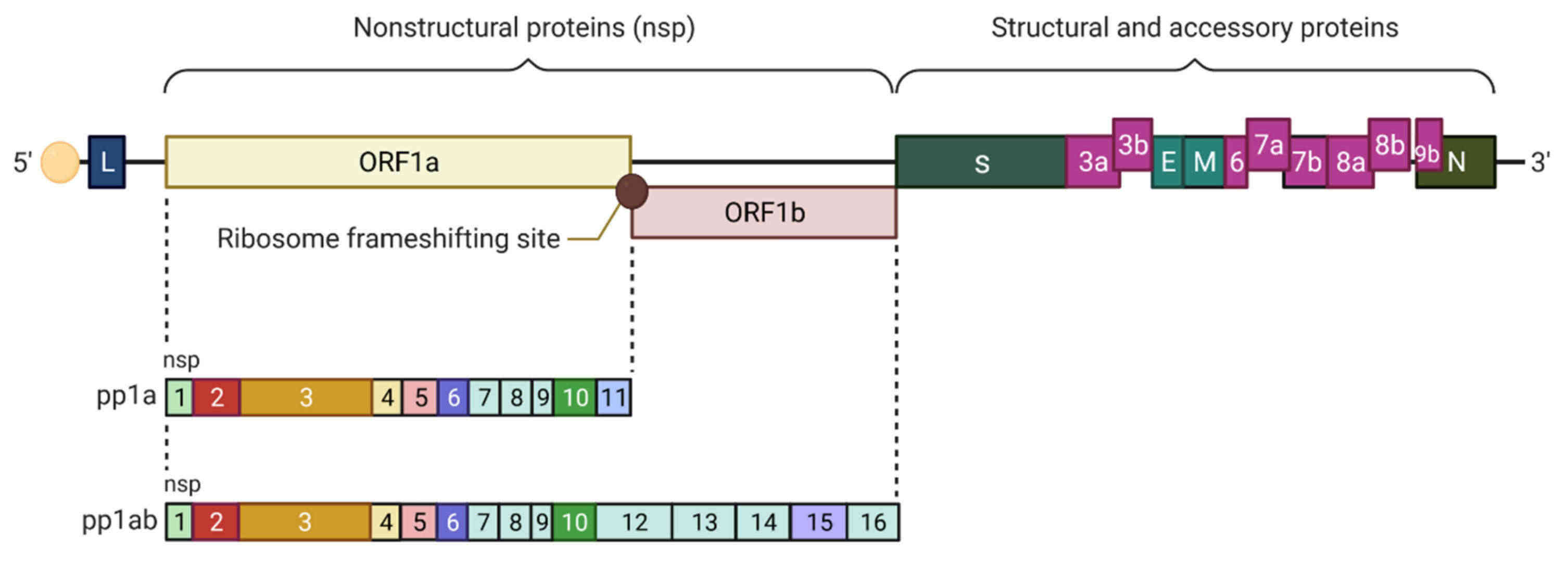 Fig.1 Genomic organization of SARS-CoV. (Sharma H N, et al., 2021)
Fig.1 Genomic organization of SARS-CoV. (Sharma H N, et al., 2021)
Accurate and timely diagnosis of SARS relies on a multi-method approach to detect active infection, past exposure, and viral presence across different stages of the disease. Key techniques include molecular diagnostics (RT-PCR) for early viral detection, serological testing (ELISA) for immune response analysis, and antigen-based methods (lateral flow assays) for rapid screening. Each method has distinct advantages and limitations, making them suitable for specific clinical and public health scenarios.
| Feature | RT-PCR | ELISA | Lateral Flow Assays (LFAs) |
| Target | Viral RNA (RdRp, N, E genes) | Antibodies (IgM/IgG) | Viral antigens (N protein) |
| Sample Type | Nasopharyngeal swab, sputum, BAL | Serum/plasma | Nasopharyngeal swab |
| Time to Result | 2–6 hours (lab-dependent) | 2–4 hours (batch processing) | 15–30 minutes |
| Sensitivity | High (90–95%) | Moderate (IgM: 70–85%; IgG: >90%) | Low to moderate (60–80%) |
| Specificity | High (>95%) | High (IgG neutralization: gold standard) | Moderate (cross-reactivity risk) |
| Stage Detected | Acute phase (early infection) | Convalescent phase (IgM/IgG, post-symptom) | Acute phase (high viral load) |
| Equipment Needed | Thermocycler, RNA extraction tools | Plate reader, washer | None (visual readout) |
| Skill Level | High (trained technicians) | Moderate (lab experience) | Low (minimal training) |
| Use Case | Confirmatory diagnosis, outbreak tracking | Serosurveillance, past exposure assessment | Rapid screening, field testing |
Severe acute respiratory syndrome (SARS) diagnosis relies on detecting viral components (direct biomarkers) and host immune responses (indirect biomarkers). Below is a categorized breakdown of critical biomarkers used in clinical and laboratory settings:
| Biomarker | Role in Diagnosis | Detection Method | Clinical Utility |
| Viral RNA | Targets RdRp, N, E genes | RT-PCR, RT-LAMP | Gold standard for acute infection. |
| Nucleocapsid (N) Protein | Highly abundant structural protein | Antigen LFAs, ELISA | Rapid screening; lower sensitivity. |
| Spike (S) Protein | Facilitates host cell entry; strain-specific | Neutralization assays, ELISA | Vaccine research/serology. |
| Biomarker | Role in Diagnosis | Detection Method | Clinical Utility |
| IgM/IgG Antibodies | IgM: Acute phase (~10 days post-onset) | ELISA, CLIA | Confirms recent/past infection. |
| Neutralizing Antibodies | Block viral infection (gold standard) | Plaque reduction assays | Vaccine efficacy studies. |
| Inflammatory Markers | Predict disease severity (e.g., cytokine storm) | ELISA, Luminex | IL-6, CRP, ferritin guide ICU care. |
The field of severe acute respiratory syndrome (SARS) diagnostics is rapidly evolving, driven by lessons from past outbreaks and the need for faster, more accurate, and accessible testing solutions. Emerging technologies aim to address key challenges like cross-reactivity, resource limitations, and early outbreak detection. Below are the most promising innovations:

Multiplex Panels for Respiratory Pathogens
Multiplex PCR panels enable simultaneous detection of SARS-CoV alongside 20+ respiratory pathogens (influenza, RSV, SARS-CoV-2) in a single test. These panels use automated, closed systems to reduce contamination risk and provide results in 1–2 hours, making them ideal for hospital outbreaks or co-infection screening.
Multiplex Panels for Respiratory Pathogens
NGS provides strain-level resolution of SARS-CoV by sequencing entire viral genomes, aiding in tracking zoonotic origins and mutations (e.g., spike protein variants). Metagenomic approaches allow unbiased pathogen detection in complex samples (e.g., wastewater, BAL fluid), crucial for identifying novel coronaviruses.
Lab-on-a-Chip & Microfluidics
These miniaturized systems integrate RNA extraction, amplification, and detection into credit-card-sized devices, enabling portable, automated SARS testing. Microfluidic PCR delivers results in <30 minutes with minimal hands-on time, ideal for emergency departments or resource-limited settings.
The fight against severe acute respiratory syndrome (SARS) relies on accurate, rapid, and portable diagnostic methods that combine gold standard methods like RT-PCR with cutting-edge innovative technologies like multiplex panels and microfluidics testing. From acute-phase detection to retrospective surveillance, these tools are critical for outbreak containment, patient management, and pandemic preparedness. Continued developments in technologies such as artificial intelligence, portable sequencing, and lab-on-a-chip systems promise faster, more decentralized testing to address emerging coronavirus threats. By integrating these advances, laboratories and public health teams can enhance the global response to a potential SARS resurgence or a novel betacoronavirus outbreak.
Alta DiagnoTech provides comprehensive IVD solutions for severe acute respiratory syndrome (SARS) diagnostics, including high-precision RT-PCR kits, rapid antigen tests, and automated serology assays to support accurate detection and outbreak response. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| PI-00121 | In vitro polymerase chain reaction (PCR) SARS-CoV-2 assay kit | Add To Cart |
| LM-QCY-0007 | SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit | Add To Cart |
| VI-QCY-0011 | Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Test Kit | Add To Cart |
| NATR-HMM-0008 | SARS-CoV-2 Novel Coronavirus Nucleic Acid Detection Reagent | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |