- Home
- Resource
- Explore & Learn
- Revolutionizing Mild Traumatic Brain Injury Diagnosis: The Game-Changing UCH-L1 and GFAP Blood Test
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Mild Traumatic Brain Injury (mTBI), often resulting from falls, sports accidents, or motor vehicle collisions, has long posed a significant diagnostic challenge. For years, computed tomography (CT) scans were the primary means of assessing mTBI. However, CT scans expose patients to ionizing radiation, carry substantial costs, and may not detect subtle injuries. The absence of a reliable, rapid, and non-invasive diagnostic alternative left a critical gap in patient care. In emergency departments, healthcare providers frequently face the dilemma of whether to subject patients to potentially unnecessary CT scans, leading to overexposure to radiation and increased healthcare expenses.
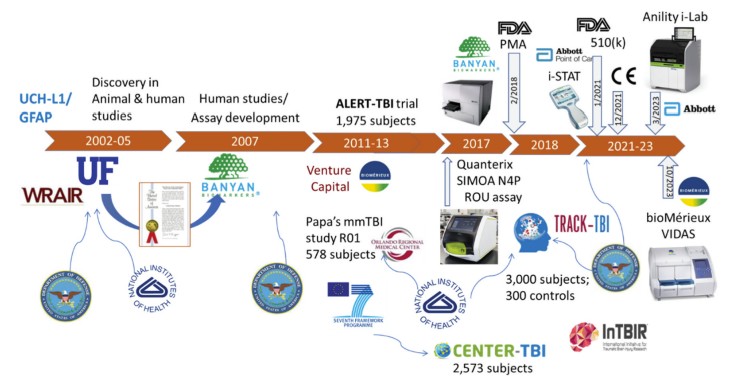 Fig.1 A brief history of UCH-L1/GFAP as TBI diagnostic biomarkers. (Kobeissy F., et al., 2024)
Fig.1 A brief history of UCH-L1/GFAP as TBI diagnostic biomarkers. (Kobeissy F., et al., 2024)
Neuronal ubiquitin C-terminal hydrolase-L1 (UCH-L1) is a protein predominantly expressed in neurons within the central nervous system. Its primary function involves the regulation of the ubiquitin-proteasome system, which is crucial for maintaining neuronal health by deubiquitinating proteins. When a mild traumatic brain injury occurs, damaged neurons release UCH-L1 into the bloodstream. In a study involving patients with mTBI, UCH-L1 levels were found to peak within hours of injury, making it an early indicator. For instance, in cases of concussions sustained during contact sports, UCH-L1 concentrations began to rise as soon as 30 minutes post-injury, providing a rapid signal of neuronal damage.
Glial fibrillary acidic protein (GFAP) is a major intermediate filament protein in astrocytes, the star-shaped glial cells that support neurons. Following a brain injury, astrocytes become activated and release GFAP into the circulation. Unlike UCH-L1, GFAP levels tend to increase more gradually, peaking around 24-48 hours after the injury. This temporal pattern makes GFAP useful for confirming injuries even when patients present later. In a cohort of mTBI patients who sought medical attention several hours after an accident, elevated GFAP levels were strongly correlated with the presence of brain lesions detected on subsequent CT scans.

Pre-Clinical Research
The journey to developing UCH-L1 and GFAP-based blood tests began with extensive pre-clinical research. Animal models, such as rats and mice, were subjected to controlled traumatic brain injuries. Through proteomic analysis of brain tissue and blood samples from these animals, researchers identified UCH-L1 and GFAP as proteins that were consistently released into the bloodstream following injury. These initial findings provided the foundation for further human-based studies.

Clinical Trials
Large-scale clinical trials were essential for validating the diagnostic utility of these biomarkers. The BANYAN and TRACK-TBI studies were pivotal in this regard. In the BANYAN study, over 1,000 patients with suspected mTBI were enrolled. Blood samples were analyzed for UCH-L1 and GFAP levels, and the results were compared with CT scan findings. The study demonstrated that patients with elevated UCH-L1 and GFAP levels were significantly more likely to have CT-detectable brain injuries. The negative predictive value of the combined biomarker test was extremely high, indicating that a negative test result was highly reliable in ruling out significant brain injury.
Reducing Unnecessary CT Scans
One of the most significant impacts of these blood tests is the reduction in unnecessary CT scans. In emergency departments, the i-STAT Alinity™ test, which measures UCH-L1 and GFAP, has been shown to decrease the rate of CT scans by up to 40%. For example, in a busy urban emergency department that implemented the test, the number of CT scans for mTBI patients dropped from an average of 120 per month to 72 per month. This not only reduces radiation exposure for patients but also frees up valuable CT scanner time for other critical cases.
Faster Diagnosis and Treatment
The point-of-care nature of tests like the i-STAT AlinityTM and BioMerieux’s VIDAS TBI test allows for rapid results. With results available in as little as 15 minutes, healthcare providers can make more informed decisions quickly. In a sports-related mTBI scenario, a player can be evaluated on the sidelines, and if the test results are negative, they can potentially return to play safely, reducing the time spent on the bench and the anxiety associated with waiting for a diagnosis.

Despite their significant advantages, UCH-L1 and GFAP blood tests have limitations. The tests are currently approved only for adults, and their use in pediatric populations remains a challenge due to differences in biomarker expression and injury mechanisms. Additionally, the tests are most effective when administered within 12 hours of injury, which may limit their utility for patients who present later. In cases of polytrauma, where there are multiple injuries, including non-brain injuries, there is a potential for false positives, as other tissues may also release proteins that could interfere with the test results.
Ongoing research aims to overcome these limitations. Studies are underway to develop pediatric-specific biomarker cut-offs for UCH-L1 and GFAP. Researchers are also exploring ways to extend the diagnostic window beyond 12 hours, perhaps by combining these biomarkers with other emerging ones. The development of multiplex tests that can measure multiple brain injury-related biomarkers simultaneously holds promise for a more comprehensive understanding of the injury and better prediction of long-term outcomes, such as the development of post-concussion syndrome or neurodegenerative diseases.
In conclusion, the UCH-L1 and GFAP blood tests have revolutionized the diagnosis of mild traumatic brain injury. These tests have filled a long-standing diagnostic void, providing a reliable, rapid, and non-invasive alternative to traditional CT scans. While challenges remain, the future of mTBI diagnosis looks promising, with continued research likely to further enhance the utility of these biomarkers and improve patient care.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |