- Home
- Resource
- Explore & Learn
- Revolutionizing Disease Detection: The Rise of Point-of-Care and Point-of-Need Diagnostics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In the realm of infectious diseases, time is often of the essence. The ability to swiftly and accurately diagnose pathogens at the point of care (POC) or point of need (PON) has become a cornerstone in managing outbreaks and preventing widespread transmission. Traditional diagnostic methods, reliant on centralized laboratories, are often hindered by long turnaround times and logistical challenges. The advent of point-of-care and point-of-need diagnostics promises to revolutionize disease detection by bringing advanced testing capabilities directly to the patient's side or outbreak location.
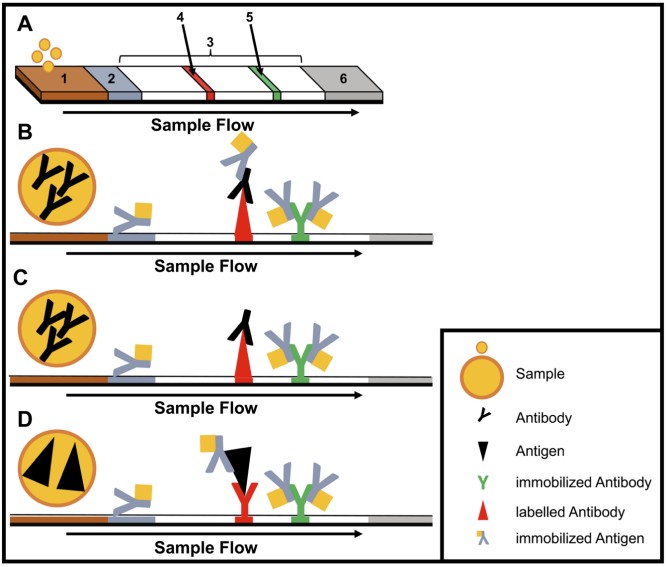 Fig.1 Structure and type of a lateral flow immunoassay. (Hansen S., et al., 2020)
Fig.1 Structure and type of a lateral flow immunoassay. (Hansen S., et al., 2020)

From Centralized Labs to Portable Solutions
Historically, diagnostic testing for infectious diseases has been confined to centralized laboratories equipped with sophisticated instruments. While these facilities offer high throughput and accuracy, they are often inaccessible in resource-limited settings or during outbreaks where immediate results are crucial. The evolution of diagnostic technologies has seen a shift towards portable, field-applicable solutions that can be deployed rapidly and operated with minimal training.

Immunodiagnostics: The Foundation of Rapid Testing
Immunodiagnostics, based on the detection of specific antibodies or antigens, have long been the backbone of rapid testing. Lateral flow immunoassays (LFIAs), for instance, are widely used for their simplicity, affordability, and rapid turnaround times. These assays, often performed in disposable cartridges, require no pipetting or washing steps and can be read visually within minutes. Multiplex LFIAs have further enhanced diagnostic capabilities by enabling the simultaneous detection of multiple targets.

Nucleic Acid Amplification: Enhancing Sensitivity and Specificity
While immunodiagnostics offer rapid results, nucleic acid amplification methods provide unparalleled sensitivity and specificity by targeting the genetic material of pathogens. Polymerase chain reaction (PCR) has been the gold standard for nucleic acid amplification, but its reliance on complex equipment and thermal cycling has limited its use in the field. Isothermal amplification techniques, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), have emerged as viable alternatives. These methods operate at a constant temperature, eliminating the need for sophisticated thermal cyclers and enabling faster results.

European Mobile Lab Project
The European Mobile Lab Project established a moving laboratory unit in Nigeria during the hemorrhagic fever outbreak. Comprising 27 boxes weighing 20-30 kg each, the unit contained over 400 equipment items needed to set up a fully functional BSL3 or BSL4 diagnostic laboratory. This mobile lab enabled rapid sample inactivation, molecular diagnostics, and antigen/antibody testing, proving invaluable in controlling the outbreak.

Mobile Suitcase Laboratory
Originally developed for avian influenza detection, the mobile suitcase laboratory was adapted for Ebola diagnosis during the West African outbreak. This compact solution consists of two trolley cases, a solar panel, a power pack, and an optional glove box. One case is used for nucleic acid extraction, while the other contains instruments for isothermal amplification or nanopore sequencing. Its portability and ease of use make it ideal for remote or resource-limited settings.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |