- Home
- Resource
- Explore & Learn
- Revolutionizing Cervical Cancer Screening: The Paperfluidic Molecular Diagnostic Chip
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Cervical cancer continues to pose a significant global health challenge, particularly in developing countries where healthcare resources are scarce. According to the World Health Organization (WHO), cervical cancer is the fourth most common cancer among women worldwide, with an estimated 604,000 new cases and 342,000 deaths in 2020 alone. The highest incidence rates are found in regions with limited access to effective screening and preventive measures.
Traditional screening methods, such as the Pap smear, have been instrumental in reducing cervical cancer mortality in developed countries. However, these methods require sophisticated laboratory infrastructure, trained personnel, and multiple clinic visits, making them impractical in low-resource settings. The sensitivity of cytology-based screening can be as low as 53%, leading to false negatives and delayed diagnoses. Visual inspection with acetic acid (VIA) has been proposed as a low-cost alternative, but its sensitivity and specificity remain suboptimal, resulting in high rates of false positives and negatives.
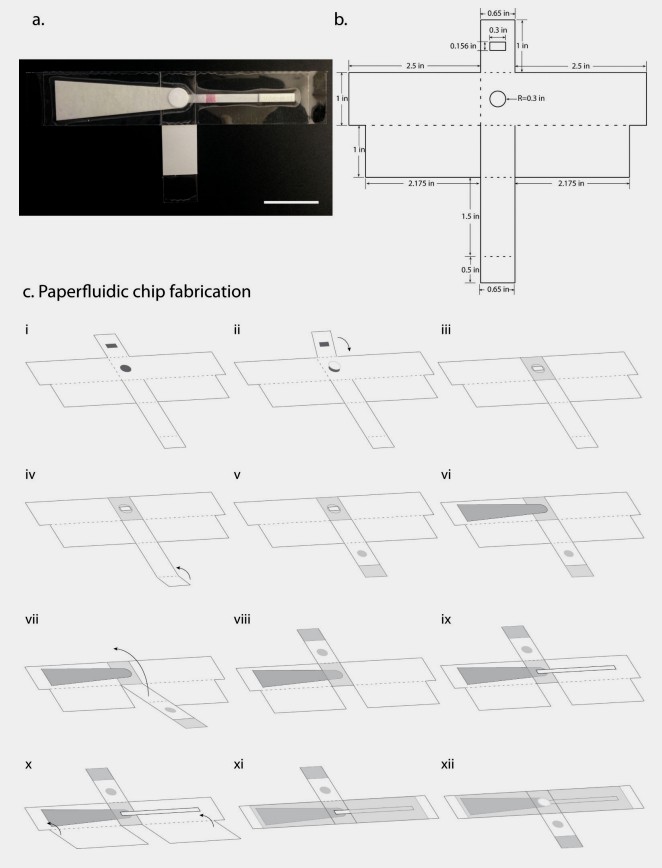 Fig.1 Paperfluidic Molecular Diagnostic Chip. (Rodriguez N. M., et al., 2016)
Fig.1 Paperfluidic Molecular Diagnostic Chip. (Rodriguez N. M., et al., 2016)
Given the limitations of existing screening methods, there is an urgent need for innovative, cost-effective, and user-friendly technologies that can be deployed in low-resource settings. Molecular diagnostics, particularly those targeting human papillomavirus (HPV), the primary etiological agent of cervical cancer, offer higher sensitivity and specificity. Over 99% of cervical cancer cases are caused by HPV, with HPV 16 being the most prevalent high-risk subtype.
HPV DNA testing has demonstrated high sensitivity (>96-100%) and specificity (>90-100%) in detecting cervical precancerous lesions. However, current HPV DNA tests require sophisticated laboratory equipment, highly trained personnel, and turnaround times ranging from hours to days. These limitations hinder their widespread adoption in resource-limited settings.
Paperfluidics, a subfield of microfluidics, leverages the unique properties of paper to create low-cost, portable, and disposable diagnostic devices. Paper's ability to passively transport fluids through capillary action eliminates the need for external pumps or complex fluid handling systems. This makes paperfluidic devices particularly suitable for point-of-care (POC) diagnostics in low-resource settings.
Several studies have reported on the successful implementation of individual steps of nucleic acid amplification tests (NAATs)—such as extraction, amplification, and detection—within paper matrices. However, integrating these steps into a single, fully automated device has been challenging.
The paperfluidic molecular diagnostic chip is a fully integrated, single-use device designed for the extraction, amplification, and detection of nucleic acids from clinical samples. The chip is fabricated using standard letter-size self-adhesive laminating sheets as the base material, providing a hydrophobic barrier that surrounds the paper components. Polyethersulfone (PES) filter paper is used for sample filtration and nucleic acid retention, while cellulose blotting paper serves as the absorbent pad.
The chip's design incorporates perforations in the adhesive sheets to enable easy folding and assembly. The sample port, created by placing a PES disc over a hole in the adhesive sheet, facilitates sample introduction and filtration. The absorbent pad, positioned directly beneath the sample port, wicks away the liquid waste, leaving the solid phase (including nucleic acids) on the PES membrane.
The nucleic acid extraction process leverages chaotropic lysis and alcohol precipitation methods. A single-use pellet of the clinical specimen is resuspended in a lysis buffer containing chaotropic agents, which disrupt cell membranes and release nucleic acids. The liquid phase wicks through the PES membrane, leaving the precipitated nucleic acids behind. Ethanol washes are then used to remove impurities, while the purified nucleic acids remain on the PES membrane.
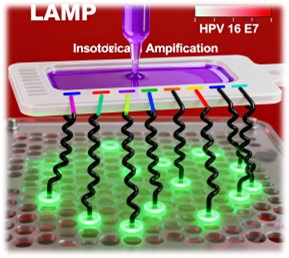
Isothermal amplification, specifically loop-mediated amplification (LAMP), is employed for the rapid and sensitive detection of HPV DNA. LAMP offers several advantages over traditional polymerase chain reaction (PCR), including the ability to amplify nucleic acids at a constant temperature, eliminating the need for thermal cyclers.
The LAMP assay developed for the paperfluidic chip targets the HPV 16 E7 gene, a key marker for cervical cancer. The assay uses a set of six primers that recognize distinct regions of the target sequence, ensuring high specificity. The LAMP reaction mix is pipetted directly onto the PES membrane, where it dissociates the nucleic acid-precipitant complexes and initiates amplification. The chip is then placed on a heat block or hot plate for 30 minutes at 63°C to facilitate amplification.
Following amplification, the presence of HPV 16 DNA is detected using a lateral flow immunochromatographic strip. The amplified products are eluted from the PES membrane by adding nuclease-free water, which wicks through the membrane and onto the lateral flow strip. The strip contains streptavidin-conjugated gold nanoparticles that bind to biotinylated primers within the amplicons. As the liquid migrates along the strip, amplicons containing the FITC-tagged primers aggregate at the anti-FITC test line, producing a visible signal. A control line confirms proper strip function.
The paperfluidic molecular diagnostic chip offers several advantages over existing cervical cancer screening methods. Its low cost, portability, and ease of use make it suitable for deployment in low-resource settings where traditional laboratory infrastructure is lacking. The fully integrated design allows for rapid, sample-to-answer testing in under an hour, facilitating immediate diagnosis and treatment decisions.
By providing highly sensitive molecular-level information on the presence of high-risk HPV strains, the chip addresses the limitations of cytology-based screening. Its potential for primary HPV screening, followed by cytology triage for positive results, aligns with current guidelines and could significantly reduce cervical cancer incidence and mortality in developing countries.
While the paperfluidic chip represents a significant advancement in cervical cancer screening, several challenges remain. Current prototypes require an external heat source for isothermal amplification, limiting their portability. Incorporating an integrated heating system, such as a battery-operated resistance heater or exothermic chemical reaction, could enhance the chip's usability in remote settings.
Further optimization of the chip design is needed to minimize user manipulation and reduce the risk of contamination. Increasing the surface area of the sample port and optimizing buffer conditions could simplify the workflow and reduce reagent volumes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
We provide molecular diagnostic kits, which are efficient tools designed for molecular biology research and clinical diagnosis and can accurately detect changes in nucleic acid (DNA/RNA) sequences, structures, or expression levels in biological samples, which help medical research such as disease diagnosis, pathogen detection, and genetic disease screening. Specifically covering the following types:

This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |