- Home
- Resource
- Explore & Learn
- Revolutionizing Allergy Testing: From Bucket Chemistry to Precision Diagnostics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Allergies are a widespread health issue, affecting millions of people worldwide. From sneezing and itching to severe anaphylactic reactions, allergic responses can significantly impact quality of life. Accurate and reliable allergy testing is crucial for diagnosing and managing these conditions. However, traditional allergy testing methods have long been criticized for their lack of precision and consistency. This article explores the latest advancements in allergy diagnostics, highlighting how modern biochemical techniques are transforming the field from the era of "bucket chemistry" to a new age of precision testing.
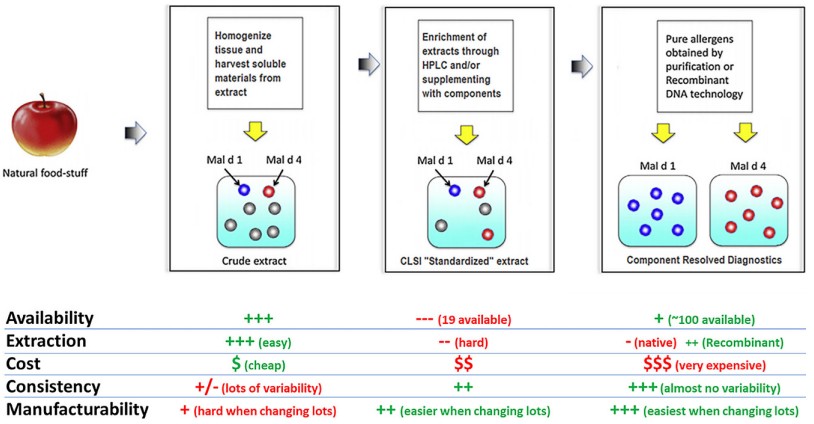 Fig.1 Crude extracts are prepared by homogenizing tissue to isolate soluble components. (Whitters E., et al., 2025)
Fig.1 Crude extracts are prepared by homogenizing tissue to isolate soluble components. (Whitters E., et al., 2025)
Traditional allergy testing, often referred to as "bucket chemistry," relies on crude allergen extracts with minimal biochemical characterization. These extracts are complex mixtures containing various allergenic and nonallergenic substances, including proteins, glycoproteins, polysaccharides, lipids, nucleic acids, and low molecular weight metabolites. The allergens themselves typically make up less than 1% of the total constituents, contributing to significant lot-to-lot variability. This variability can lead to inconsistent test results, making it challenging for clinicians to accurately diagnose and manage allergies.
To overcome these limitations, modern allergy testing must tightly control the sourcing, analysis, and performance testing of allergen extracts. Contemporary biochemical techniques offer a more precise and reliable approach to characterizing allergens. By using advanced methods such as polyacrylamide gel electrophoresis (PAGE), high-performance liquid chromatography (HPLC), and Western blot analysis, manufacturers can better understand the composition and potency of allergen extracts. This, in turn, leads to more accurate and consistent test results that better reflect a patient's clinical status.
Table 1. Comparison of traditional and modern allergy testing methods. (Whitters E., et al., 2025)
| Aspect | Traditional Methods | Modern Methods |
| Extract Composition | Crude mixtures with minimal characterization | Biochemically enriched or purified components |
| Consistency | High lot-to-lot variability | Enhanced consistency and uniformity |
| Sample Volume Required | High (1-2 mL serum) | Low (e.g., 4µL for NOVEOS) |
| Testing Approach | Singleplex (one allergen per test) | Singleplex and multiplex (multiple allergens) |
| Technologies Used | Basic biochemical techniques | Advanced techniques (SDS-PAGE, HPLC, CRD) |
| Clinical Relevance | Ambiguous results | More precise and clinically relevant results |
One significant advancement in allergy diagnostics is the shift from crude extracts to biochemically enriched extracts and component-resolved diagnostics (CRD). Biochemically enriched extracts focus on isolating and concentrating specific allergenic proteins from natural sources, resulting in a more uniform and controlled preparation. CRD, on the other hand, involves using purified individual proteins, either as native proteins enriched to homogeneity or as recombinant versions. These approaches enhance precision and accuracy, allowing for a more reliable identification of allergens responsible for immune responses.
To ensure that allergen extracts meet stringent performance requirements, diagnostic manufacturers must commit to extensive testing at every stage of production. This includes raw material sourcing, qualification, in-process testing, and final quality control release. Analytical techniques such as SDS-PAGE and HPLC are used to assess the integrity of extracts, while performance testing evaluates how allergens will behave in clinical settings. Every lot should be tested against published performance criteria, including method comparison, limit of blank, assay precision, and clinical concordance. This rigorous approach ensures that allergen extracts are consistent, reliable, and clinically relevant.


Traditional singleplex assays, such as ImmunoCAP™, IMMULITE®, and HYTEC-288™, measure allergen-specific IgE levels using a single allergen extract or component per test. While effective, these methods require significant serum volumes and can be time-consuming. Recent advancements have led to the development of more efficient singleplex systems like the NOVEOS® immunoanalyzer, which uses chemiluminescence and microparticle technology to achieve accurate results with minimal sample volume (as low as 4µL per test). This technology is particularly beneficial for pediatric patients and enables the use of microsampling techniques.
Multiplex assays represent another significant advancement in allergy diagnostics. These assays, such as the Immuno Solid-Phase Allergen Chip (ISAC™), Allergy Explorer (ALEX), and Microtest, can simultaneously detect IgE specific to multiple allergens in a single test. By using chip-based microarrays or bead-based arrays, these assays require less serum volume and can provide comprehensive results quickly. However, multiplex assays may exhibit reduced sensitivity for low-level IgE detection due to the limited amount of allergen conjugated to the solid phase. Despite this, multiplex assays offer significant advantages in terms of sample efficiency and cost-effectiveness, especially when testing for sensitization to multiple allergens.
The integration of modern biochemical techniques and advanced technologies is transforming allergy diagnostics. By moving away from traditional "bucket chemistry" methods, diagnostic manufacturers can provide more precise, consistent, and clinically relevant test results. The adoption of biochemically enriched extracts, component-resolved diagnostics, and advanced singleplex and multiplex assays will continue to enhance the accuracy and efficiency of allergy testing. As these technologies become more widespread, clinicians will have better tools to diagnose and manage allergic conditions, ultimately improving patient outcomes.
The field of allergy diagnostics is undergoing a revolutionary transformation. By leveraging modern biochemical techniques and advanced technologies, diagnostic companies are moving beyond the limitations of traditional "bucket chemistry" methods. The future of allergy testing lies in precision diagnostics, where allergen extracts are carefully characterized, and tests are rigorously validated to ensure consistency and reliability. As these advancements continue to unfold, we can expect more accurate diagnoses, better management of allergic conditions, and improved quality of life for millions of allergy sufferers worldwide.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |