- Home
- Resource
- Explore & Learn
- Reimagining Health: The Future of Point-of-Care Diagnostics for Humans, Animals, and Plants
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
The rapid evolution of diagnostics technology has brought about significant changes in healthcare, agriculture, and environmental monitoring. While traditional laboratory-based diagnostics have played an essential role in identifying diseases and pathogens, they are often limited by time, cost, and accessibility. In contrast, point-of-care testing (POCT) has emerged as a game-changer, providing faster, more accessible, and cost-effective solutions for disease detection. As the world moves toward a more interconnected and proactive approach to health, the need for diagnostic systems that span across humans, animals, and plants has never been more pressing.
The concept of "One-Health", which emphasizes the interconnectedness of human, animal, and environmental health, is at the forefront of these advancements. With the rise of zoonotic diseases, plant pathogens, and environmental threats, the integration of advanced diagnostics across species is a crucial step in preventing large-scale health crises. This article delves into the future of point-of-care diagnostics, focusing on the breakthrough technologies, such as CRISPR and SiMoT, that are transforming how we approach disease detection and management across various sectors.
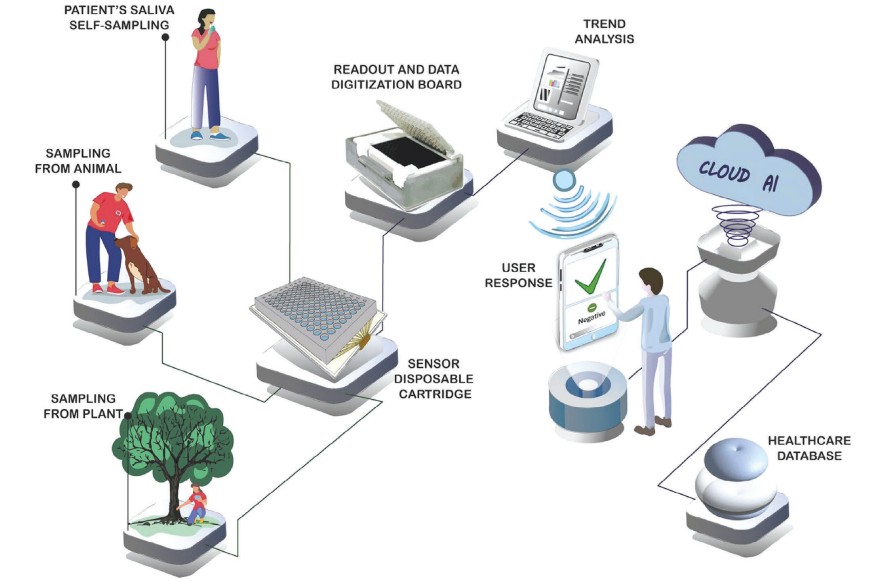 Fig.1 The vision of the screening with a POCT technology in a one-healthcare ecosystem. (Macchia E., et al., 2024)
Fig.1 The vision of the screening with a POCT technology in a one-healthcare ecosystem. (Macchia E., et al., 2024)
POCT refers to diagnostic tests conducted at or near the site of patient care, whether at home, in a clinic, or in remote areas. The primary advantage of POCT is its ability to provide quick, on-site results, enabling faster decision-making for patient management and disease intervention. Traditionally, diagnostic tests were confined to laboratories due to the need for specialized equipment and expert personnel. However, advancements in technology have led to the development of portable devices capable of performing highly accurate tests with minimal training.
Early POCT devices, such as glucose meters, revolutionized the management of chronic diseases. Today, these technologies are expanding to detect a broad spectrum of diseases and conditions, including infectious diseases, cancer, and neurological disorders. These systems, often handheld and ultra-portable, can provide results within minutes, reducing the need for expensive and time-consuming laboratory procedures.
In POCT, the key challenge is to maintain high sensitivity and specificity while ensuring the device remains affordable, portable, and easy to use. Sensitivity refers to the ability of a test to correctly identify individuals who have a disease (true positives), while specificity refers to the test's ability to correctly identify those without the disease (true negatives). Achieving high diagnostic sensitivity and specificity is critical to minimizing false positives and false negatives, which can lead to unnecessary treatments or missed diagnoses.
Recent advancements have enabled the development of POCT systems that operate at the single-molecule detection level, offering unprecedented sensitivity. This is crucial for early-stage disease detection, where biomarkers are present in very low concentrations. Technologies such as CRISPR-based assays and SiMoT devices are now pushing the limits of detection to the single-molecule threshold, offering the potential for highly accurate diagnostics with minimal sample volumes.

The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system, originally discovered as part of the bacterial immune system, has become a cornerstone of modern molecular diagnostics. CRISPR/Cas9 technology allows for precise targeting of specific DNA sequences, making it ideal for detecting genetic markers associated with various diseases.
In diagnostics, CRISPR is used in conjunction with guide RNAs that direct the Cas9 protein to specific genetic sequences in the sample. Once the target sequence is bound, the Cas9 protein introduces a double-stranded break in the DNA, triggering a detectable signal, such as fluorescence or color change. The ability of CRISPR-based biosensors to achieve fM (femtomolar) to nM (nanomolar) sensitivity has made it a powerful tool in the detection of viral infections, genetic mutations, and other biomarkers associated with diseases such as cancer, Alzheimer's, and COVID-19.
Despite its promise, CRISPR-based diagnostics often require signal amplification techniques to achieve single-molecule detection limits. This is because the CRISPR/Cas system alone cannot generate a strong enough signal to detect one molecule in a large sample volume. Amplification methods, such as polymerase chain reaction (PCR) or isothermal amplification, are integrated with CRISPR assays to increase the signal strength, making them suitable for low-concentration biomarkers.
One of the major advantages of CRISPR-based POCT systems is their versatility. By simply altering the guide RNA or the detection system, CRISPR can be tailored to detect a wide variety of pathogens and biomarkers. This flexibility makes CRISPR an invaluable tool in the One-Health approach, where the need to diagnose diseases across species—humans, animals, and plants—is essential.
SiMoT (Single-Molecule with a Large Transistor) represents another major advancement in POCT technology. SiMoT devices utilize bioelectronic sensors that detect biomarkers at the single-molecule level, directly from peripheral fluids such as blood, saliva, or plant sap. These devices are capable of achieving extremely high sensitivity without the need for complex amplification processes, making them faster and more cost-effective than traditional methods.
The core principle behind SiMoT technology is the use of biofunctionalized electrodes that capture specific proteins or nucleic acids. When these targets bind to the sensor, they cause a measurable shift in the electrochemical properties of the sensor, generating a signal that can be detected. SiMoT devices can detect biomarkers such as proteins, antigens, and DNA/RNA sequences, providing a versatile platform for a wide range of diagnostic applications.
SiMoT devices are designed to operate in ultra-portable, handheld formats, making them ideal for use in resource-limited settings or for at-home diagnostics. For instance, SiMoT's single-sensor device has been used to detect SARS-CoV-2 in patient saliva samples with remarkable sensitivity, achieving detection limits as low as one molecule per 0.1 mL of sample. Furthermore, the SiMoT platform is scalable, with the potential to be expanded into multiplexed 96-sensor arrays, allowing for the simultaneous detection of multiple biomarkers from a single sample.
SiMoT's ability to detect both proteins and nucleic acids in the same biofluid sample is a significant advantage. This feature eliminates the need for separate tests for different types of biomarkers, streamlining the diagnostic process and reducing overall costs. The SiMoT platform is also highly adaptable, as the recognition elements—such as antibodies or oligonucleotide probes—can be easily modified to detect different pathogens or disease markers. This makes SiMoT an ideal tool for diseases that affect multiple species, including zoonotic diseases that can spread between animals and humans.

The One-Health concept emphasizes the interconnectedness of human, animal, and environmental health, recognizing that diseases can spread across species and ecosystems. This approach has gained increasing importance in the context of emerging zoonotic diseases, such as COVID-19, which highlight the need for integrated diagnostic solutions.
POCT technologies like CRISPR and SiMoT offer a unique opportunity to bridge the gap between human, animal, and plant health by providing rapid, reliable diagnostics across all three domains. For instance, SiMoT has been used to detect plant pathogens, such as the Xylella fastidiosa bacterium in olive sap, as well as animal disease markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6) in livestock. These technologies can help farmers, veterinarians, and environmental scientists monitor the health of animals and plants, preventing the spread of diseases before they reach epidemic proportions.
In addition to disease detection, these technologies can be integrated into environmental monitoring systems to track the health of ecosystems. By detecting pathogens in soil, water, or air samples, POCT systems can identify potential threats to biodiversity and ecosystem stability, enabling early intervention to mitigate damage.

Despite their immense potential, several challenges remain in bringing POCT technologies to the market. One of the primary hurdles is ensuring that these devices are both cost-effective and scalable. While technologies like SiMoT and CRISPR-based diagnostics are still in the research phase, their commercial viability depends on the development of efficient manufacturing processes that can produce devices at a large scale.
Regulatory approval is another key consideration. POCT technologies must undergo rigorous testing to ensure that they meet the necessary standards for accuracy, reliability, and safety. The World Health Organization's REASSURED criteria for POCT systems—affordability, sensitivity, specificity, user-friendliness, and rapid results—provide a framework for evaluating these technologies and ensuring they meet the needs of global health systems.
Ethical concerns, such as data privacy and equitable access, also need to be addressed. As these technologies are integrated into health systems worldwide, it is essential to ensure that they are accessible to all populations, particularly those in low-resource settings.
The future of diagnostics is bright, with technologies like CRISPR and SiMoT leading the way in transforming how we detect and manage diseases. By enabling point-of-care testing at the single-molecule level, these innovations have the potential to revolutionize healthcare, agriculture, and environmental monitoring. The integration of these technologies into a One-Health framework will allow for more effective disease surveillance, early intervention, and a proactive approach to health that spans across species.
As these technologies continue to evolve, the focus will be on scaling production, securing regulatory approvals, and ensuring equitable access to these life-saving tools. With the continued development of POCT technologies, we are on the brink of a new era in diagnostics—one where health is reimagined through the power of advanced technology, bridging the gap between humans, animals, and plants in the pursuit of a healthier world for all.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |