- Home
- Resource
- Explore & Learn
- Regenerative Therapies for Intervertebral Disc Degeneration: The Role of Allogeneic FE002-Disc Cells
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Intervertebral disc (IVD) degeneration remains one of the leading causes of chronic low back pain (LBP), a condition that severely impacts quality of life and is a leading cause of disability worldwide. Despite significant advancements in both conservative and surgical treatments, these therapies often provide only temporary relief and fail to address the underlying degenerative processes. Regenerative medicine, particularly cell-based therapies, has emerged as a promising approach to treat IVD degeneration. Among the various strategies, the use of allogeneic FE002-Disc progenitor cells in IVD regeneration is showing significant potential. This article explores the role of FE002-Disc cells in regenerative therapies for IVD degeneration, focusing on their preclinical validation, functional optimization, and the challenges and benefits of their use.
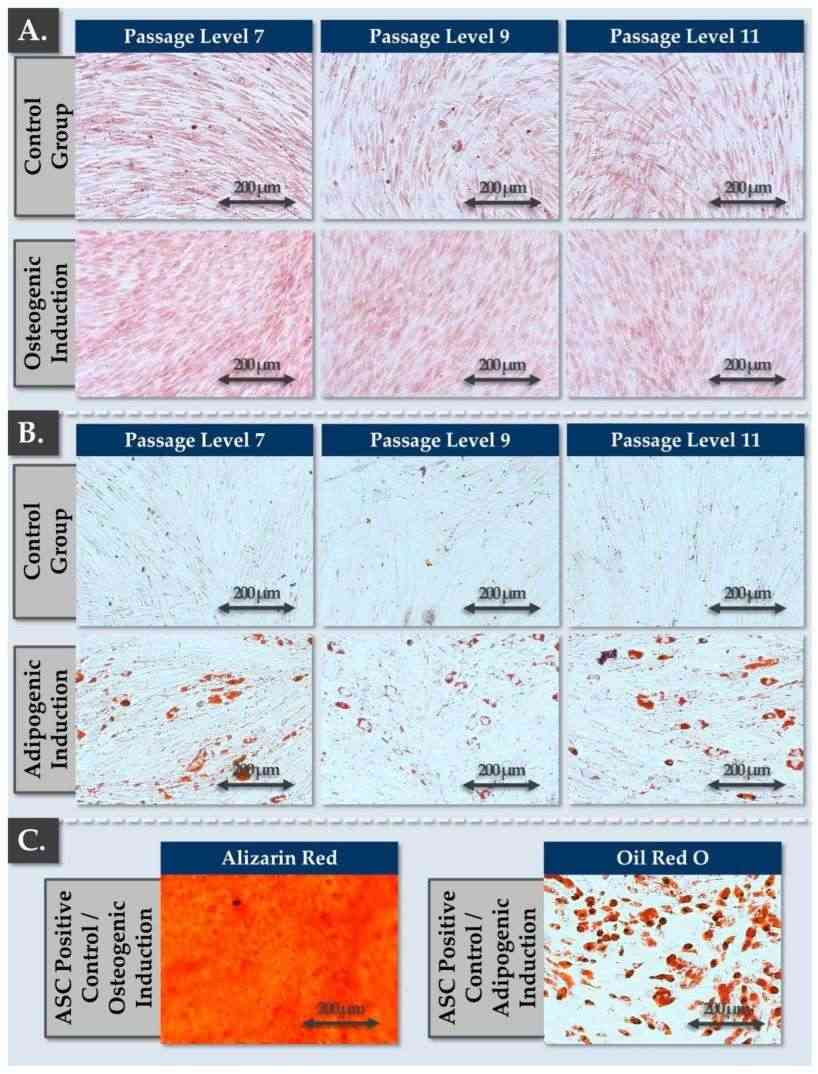 Fig.1 Results of iterative FE002-Disc progenitor cell phenotype plasticity assessments in chemical osteogenic and adipogenic induction studies. (Jeannerat A., et al., 2024)
Fig.1 Results of iterative FE002-Disc progenitor cell phenotype plasticity assessments in chemical osteogenic and adipogenic induction studies. (Jeannerat A., et al., 2024)
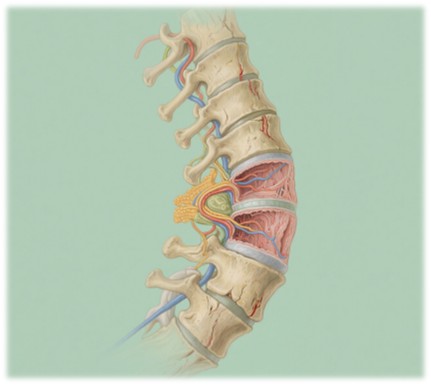
The intervertebral disc functions as a cushion between the vertebrae of the spine, absorbing shock and providing mobility. It consists of three main components: the outer annulus fibrosus (AF), the inner nucleus pulposus (NP), and the cartilaginous endplates that connect the disc to the vertebrae. IVD degeneration occurs when these structures break down, leading to the loss of disc height, reduced flexibility, and the formation of disc bulges or herniations. This degeneration is typically associated with aging, mechanical stress, and genetic factors.
The NP, rich in extracellular matrix (ECM) components such as aggrecan and collagen II, plays a central role in maintaining the mechanical properties of the disc. However, the avascular and aneural nature of the IVD makes it particularly susceptible to injury and degeneration. With the loss of key ECM components and the decrease in cellular regeneration capacity, the IVD's ability to perform its shock-absorbing function diminishes, leading to pain, stiffness, and eventually, loss of mobility.
Treatment for IVD degeneration primarily involves conservative methods such as pain management, physiotherapy, and lifestyle modifications. However, these approaches do not address the underlying degenerative process. In cases where these treatments fail, surgical interventions like spinal fusion or disc replacement are considered. While effective in relieving pain, these surgeries often lead to adjacent segment degeneration, creating new problems in the surrounding discs.
The long-term limitations of these therapies have driven interest in regenerative approaches, particularly cell-based therapies. These therapies aim to repair, regenerate, or replace damaged tissue, potentially restoring disc function and reducing the need for invasive surgical interventions.
Regenerative medicine holds the promise of using biologics, including cells, to restore the normal structure and function of the IVD. One of the most promising avenues involves the use of allogeneic progenitor cells. These cells are derived from healthy donors, eliminating the need for patient-specific biopsies. Allogeneic cell therapies offer several advantages, including the ability to produce a consistent and scalable product, which is not influenced by donor-to-donor variability or patient-specific factors.
Among the various allogeneic cell sources, FE002-Disc cells, derived from human fetal progenitor tissue, show significant potential for IVD regeneration. These cells have been demonstrated to possess high chondrogenic potential, making them an ideal candidate for disc repair. FE002-Disc cells are capable of producing key ECM components such as collagen II and aggrecan, both of which are critical for maintaining the structural integrity and function of the IVD.
The FE002-Disc cell line is established from fetal progenitor tissue sourced under stringent ethical and medical guidelines. These cells are cryopreserved at early passage levels to ensure their functional integrity. Once sourced, the cells undergo rigorous quality control and qualification processes to confirm their safety, purity, and functionality.
A critical aspect of the FE002-Disc cells' development is the manufacturing process. The cells are expanded in culture and subjected to specific conditions that promote their differentiation into discogenic cell types. One of the key steps in their preparation involves the use of a chondrogenic induction medium, which enhances their ability to synthesize ECM components. Furthermore, optimizing the spheroid formation process allows for the creation of 3D cellular structures that mimic the native disc environment, promoting cell survival and integration after implantation.
Enhancing Cell Resilience in Hypoxic and Inflammatory Environments
One of the key challenges in IVD regeneration is the harsh implantation environment. The IVD is avascular, and cells in this region are exposed to low oxygen levels (hypoxia) and inflammatory cytokines, which can limit cell survival and function. FE002-Disc cells have shown remarkable resilience under these adverse conditions.
In vitro studies have demonstrated that these cells maintain their viability and function when cultured in hypoxic conditions, simulating the low-oxygen environment of the IVD. Moreover, the cells exhibit robust chondrogenic differentiation, producing collagen II and glycosaminoglycans (GAGs), even when exposed to inflammatory stimuli like TNF-α. This resilience is critical for ensuring the success of cell-based therapies in the IVD, as it enables the transplanted cells to survive and thrive in the degenerated disc environment.
Spheroid Formation for Enhanced Therapeutic Efficacy
A major innovation in cell-based therapies for IVD regeneration is the use of cell spheroids. These three-dimensional aggregates of cells provide a more native-like environment, supporting better cell-cell interactions and ECM deposition. In the case of FE002-Disc cells, spheroid formation not only protects the cells from the harsh implantation environment but also promotes their chondrogenic differentiation.
The spheroid formulation process has been optimized to enhance the size, functionality, and stability of the spheroids. FE002-Disc cells cultured under hypoxic conditions and induced with chondrogenic medium have been shown to form larger and more robust spheroids, with enhanced ECM production. These spheroids are capable of producing high levels of collagen II and GAGs, which are critical for restoring the structural and functional properties of the IVD.
Furthermore, the stability of the spheroids post-cryopreservation and lyophilization has been demonstrated, providing the potential for off-the-shelf therapies. The ability to store spheroids in a stable form significantly increases the feasibility of cell-based IVD therapies, making them more accessible and scalable for clinical use.
Before progressing to clinical trials, it is essential to thoroughly evaluate the safety of the FE002-Disc cells. This involves assessing their potential for tumorigenicity, as well as confirming their limited proliferative lifespan to prevent uncontrolled cell growth.
Safety assays, such as the soft agar colony formation test and β-galactosidase staining for senescence, have demonstrated that FE002-Disc cells do not exhibit tumorigenic behavior. Moreover, these cells undergo senescence after several passages, ensuring that they do not maintain an indefinite proliferative capacity, a common risk associated with cell-based therapies.

The functional efficacy of FE002-Disc cells in IVD repair has been demonstrated through various in vitro assays, where they have been shown to produce key ECM components, including collagen II and aggrecan. These components are crucial for restoring the structural integrity and function of the IVD. Moreover, when cultured in 3D spheroid forms, FE002-Disc cells exhibit enhanced chondrogenic differentiation, producing a cartilage-like matrix that mimics the natural composition of the NP.
Preclinical studies have also highlighted the cells' ability to integrate into the degenerated disc environment, further supporting their potential for IVD repair. The combination of their chondrogenic potential and resilience to harsh conditions makes FE002-Disc cells a promising candidate for clinical translation.
The promising preclinical data on FE002-Disc cells pave the way for their clinical investigation. Several clinical trials are already underway using various progenitor cells for IVD regeneration, and FE002-Disc cells are expected to enter clinical studies in the near future. These trials will be crucial for assessing the long-term safety and efficacy of these cells in human patients.
While the preclinical results are promising, several challenges remain in translating FE002-Disc cell-based therapies to the clinic. One of the key hurdles is optimizing the manufacturing process to ensure scalability and reproducibility. Additionally, regulatory hurdles must be navigated, including ensuring the safety and efficacy of allogeneic cell-based products. Despite these challenges, the potential benefits of FE002-Disc cells, including their scalability and ability to regenerate IVD tissue, make them a compelling option for IVD repair.
Looking ahead, the integration of FE002-Disc cells into regenerative therapies for IVD degeneration could revolutionize the treatment of chronic LBP. By restoring the structural integrity and function of the IVD, these therapies could offer long-term relief for patients suffering from degenerative disc disease. Moreover, the continued development of allogeneic cell-based therapies, along with advances in tissue engineering and 3D printing, could further enhance the efficacy of these treatments.
In conclusion, the use of allogeneic FE002-Disc progenitor cells represents a promising approach to IVD regeneration. With ongoing optimization of spheroid formation, resilience testing, and manufacturing processes, these cells have the potential to significantly improve the treatment of IVD degeneration and chronic LBP, offering a more effective, less invasive alternative to traditional therapies.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |