- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Rabies Detection Decoded: Laboratory Protocols for Timely Diagnosis and Surveillance
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Rabies, a fatal zoonotic disease with nearly 100% mortality, demands accurate and timely diagnosis to guide life-saving interventions and public health responses. This resource provides a systematic overview of current and emerging rabies diagnostic approaches, covering viral antigen detection, molecular RNA analysis, and antibody testing (RFFIT, ELISA), to help laboratories select optimal methods based on clinical context, infrastructure, and testing objectives.
Rabies is a deadly zoonotic viral disease caused by Lyssavirus infection, transmitted primarily through bites of infected animals (dogs, bats, raccoons). With a near 100% fatality rate in symptomatic cases, it causes ~59,000 deaths annually (mostly in Asia/Africa). The virus targets the CNS, leading to fatal encephalitis characterized by hydrophobia, agitation, and paralysis. Timely and accurate rabies diagnosis is critical for initiating life-saving post-exposure prophylaxis, confirming clinical cases, and implementing effective public health interventions to prevent transmission.
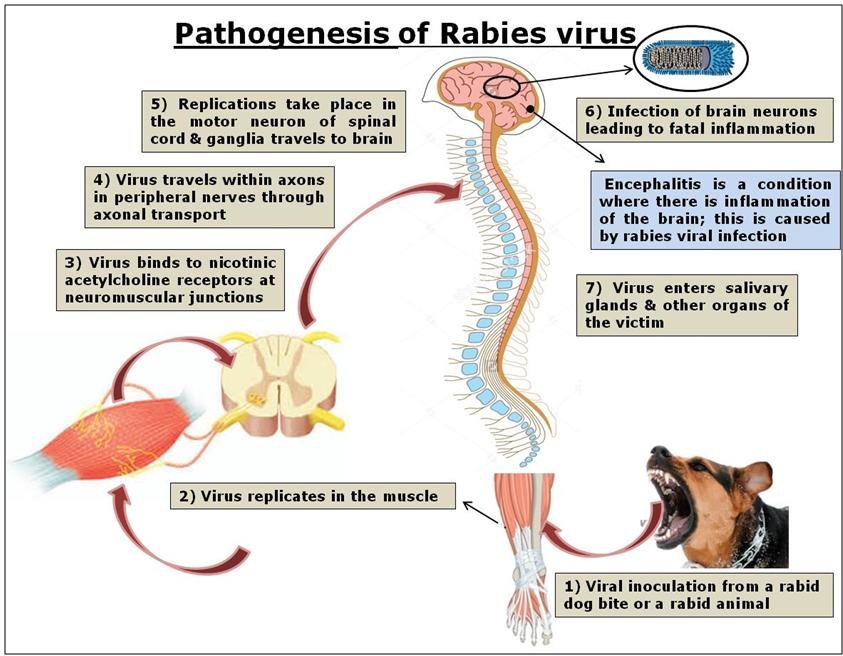 Fig.1 Pathogenesis of rabies. (Sunil Abraham, et al., 2017)
Fig.1 Pathogenesis of rabies. (Sunil Abraham, et al., 2017)
Detecting rabies viral antigens is essential for post-mortem confirmation and rapid field screening, offering direct evidence of infection. Two primary methods balance accuracy, infrastructure requirements, and operational speed to meet different diagnostic needs.
The DFA test, endorsed by WHO/OIE, detects rabies antigens in brain tissue using fluorescent-labeled antibodies. With ~99% sensitivity and specificity, it remains the gold standard for post-mortem diagnosis. However, it requires fresh tissue samples, BSL-3+ facilities, and skilled technicians, limiting its use to well-equipped laboratories.

These point-of-care tests provide results in 15–30 minutes, detecting viral antigens in saliva or brain homogenates. While useful for field surveillance in resource-limited settings, their lower sensitivity (~60%) makes them unsuitable for definitive diagnosis. They serve best as screening tools before confirmatory PCR or DFA testing.
Molecular detection of rabies viral RNA enables early and highly sensitive diagnosis, crucial for timely intervention and surveillance. Two primary approaches, RT-qPCR and isothermal amplification (RT-LAMP/RPA), offer distinct advantages in accuracy, speed, and field applicability, addressing different diagnostic needs. Below, we analyze their methodologies, strengths, and limitations.
RT-qPCR is the gold standard for ante-mortem rabies diagnosis, targeting conserved viral genes (e.g., N gene) with ~95% sensitivity in saliva, CSF, or skin biopsies. It provides quantitative results within 2–6 hours but requires thermocyclers and lab infrastructure, limiting use in resource-limited settings.
These methods amplify RNA at constant temperatures, eliminating the need for thermocyclers. RT-LAMP (60 minutes) and RPA (20–40 minutes) are field-deployable, ideal for decentralized testing. While slightly less sensitive (~90%) than RT-qPCR, they bridge gaps in remote areas lacking advanced lab facilities.
Detecting rabies-specific antibodies is essential for monitoring vaccine response, assessing potential immunity, and investigating rare survival cases, though it has limited utility for acute diagnosis due to delayed antibody production.
Rapid Fluorescent Focus Inhibition Test (RFFIT)
The RFFIT is the gold-standard functional assay for detecting rabies virus neutralizing antibodies (VNAs) in serum or CSF. This cell-based test quantifies the ability of patient antibodies to inhibit live rabies virus infection, providing critical data for post-vaccination immunity verification and abortive infection investigations.
ELISA (IgG/IgM)
Rabies ELISA systems use purified viral antigens (e.g., glycoprotein G) to detect virus-binding antibodies (IgG/IgM), providing a high-throughput alternative to RFFIT. These 96-well plate assays are safer and faster (4-6 hours), making them ideal for large-scale serological surveillance and vaccine immunogenicity testing.
The future of rabies diagnostics is shifting toward decentralized, rapid, and highly accurate solutions to address current limitations in field applicability and early detection. Emerging technologies like next-generation sequencing promise equipment-free, strain-specific detection within minutes, while AI-powered mobile microscopy could automate DFA interpretation in low-resource settings. Multiplex point-of-care molecular platforms integrating rabies with other zoonotic pathogens (e.g., Nipah, Lassa) are under development, alongside non-invasive saliva tests to replace invasive biopsies. These innovations aim to bridge critical gaps in PEP decision-making, global surveillance, and the "Zero by 30" elimination campaign, though challenges remain in cost, validation, and implementation.
Alta DiagnoTech offers a comprehensive portfolio of IVD solutions for rabies diagnosis, including high-sensitivity antigen detection kits, molecular RNA test kits, and validated antibody assay kits, supporting accurate detection across all stages of infection. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| VT-QCY-0039 | Human Rabies Virus (RV) Antibody (IgG) ELISA Kit | Add To Cart |
| EK-YJL-0084 | Human Rabies Virus (RV) Antibody (IgG) ELISA Kit | Add To Cart |
| EK-YJL-1597 | Dog Rabies Virus (RV) Antibody (IgG) ELISA Kit | Add To Cart |
| NATR-HMM-0078 | Rabies Virus (RV) Nucleic Acid Detection Reagent (RT-PCR) | Add To Cart |
| NATR-HMM-0080 | Rabies Virus Street Strain (RV-W) Nucleic Acid Detection Reagent (RT-PCR) | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |