- Home
- Resource
- Disease Diagnosis
- Endocrine Diseases
- Precision in Practice: The Evolving Role of Biomarkers in Male Hypogonadism Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Male hypogonadism is a clinical syndrome caused by insufficient testosterone production, encompassing multiple aspects including sexual function, physiology, metabolism, and mental health. This resource will guide you through basic biomarker cascades, advanced detection methods for complex cases, and future directions in diagnostics.
Male hypogonadism is an endocrine disorder characterized by the body's inability to produce sufficient levels of testosterone, the primary male sex hormone, due to impaired function of the testes (primary) or the hypothalamic-pituitary regulatory system (secondary). This deficiency manifests through a diverse constellation of symptoms affecting multiple organ systems, including sexual dysfunction (low libido, erectile dysfunction), reduced physical vitality (fatigue, loss of muscle mass), metabolic alterations, and psychological changes (low mood, irritability). An accurate diagnosis, therefore, relies on a critical integration of consistent clinical features with precise laboratory confirmation of low testosterone and the appropriate biochemical investigations to determine the underlying etiology.
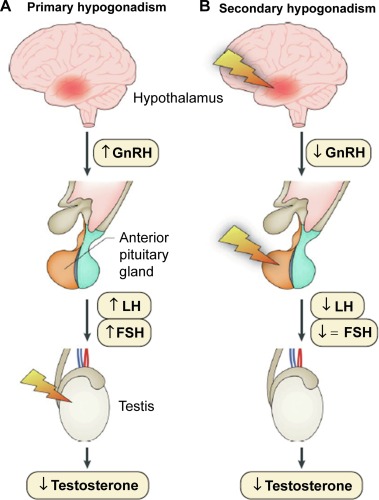 Fig.1 Causes of hypogonadism. (Salonia, Andrea, et al., 2019)
Fig.1 Causes of hypogonadism. (Salonia, Andrea, et al., 2019)
The definitive diagnosis of male hypogonadism is anchored in the precise measurement of a few key hormones. These cornerstone biomarkers function as an integrated system to first confirm the presence of testosterone deficiency and then crucially identify its origin, guiding all subsequent management decisions. Their accurate interpretation within a strict clinical and pre-analytical framework is fundamental.
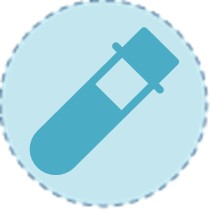
Serum Total Testosterone
Serum total testosterone measurement is the mandatory initial screening test. It requires meticulous protocol, with blood draws performed before 10:00 AM on at least two separate days to account for circadian rhythm and ensure a true baseline. A consistently low level confirms biochemical hypogonadosis, but this result alone is insufficient to complete the diagnosis or determine the cause.
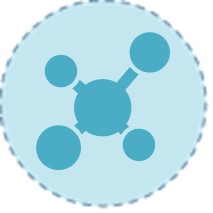
Luteinizing Hormone (LH) & Follicle-Stimulating Hormone (FSH)
Following a confirmed low testosterone, measurement of pituitary gonadotropins (LH and FSH) is the essential next step. These hormones provide the critical etiological classification: elevated levels indicate primary hypogonadism (testicular failure), while low or inappropriately normal levels point to secondary hypogonadism (a disorder of the hypothalamus or pituitary). This distinction directs the entire subsequent diagnostic workup.
Advanced biomarker assessments are employed to refine the diagnosis of male hypogonadism in complex clinical scenarios where standard tests (total testosterone, LH, FSH) yield ambiguous or conflicting results. These specialized tests provide a more nuanced view of androgen status and help pinpoint specific underlying etiologies, moving beyond simple confirmation to enable personalized management.
When total testosterone measurement may be misleading—such as in conditions that alter sex hormone-binding globulin (SHBG) levels like obesity, type 2 diabetes, aging, or liver disease—direct measurement of free testosterone (via equilibrium dialysis, the gold standard) or calculation of bioavailable testosterone becomes critical. This provides a more accurate reflection of the physiologically active hormone fraction available to tissues.


The diagnostic landscape of male hypogonadism is expanding beyond the classic hypothalamic-pituitary-gonadal axis, with research actively exploring novel biomarkers and technological advances. Key future directions include the adoption of liquid chromatography-tandem mass spectrometry (LC-MS/MS) as a more specific reference method for steroid hormone profiling, the investigation of inflammatory and metabolic markers (like leptin and adiponectin) to understand the condition's interplay with obesity and metabolic syndrome, and the refined use of genetic testing to identify underlying hereditary causes, all aiming to enable a more precise, etiologically informed, and personalized diagnostic approach.
Alta DiagnoTech offers a comprehensive portfolio of in vitro diagnostic (IVD) solutions for the precise and stratified diagnosis of male hypogonadism. Our assays support the complete clinical pathway—from the initial screening of testosterone and gonadotropins for confirmation and classification, to advanced assessments of bioactive hormone fractions and specific etiologies. These reliable tools provide the foundational and specialized data required to navigate from clinical suspicion to personalized management decisions. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| Total Testosterone Immunoassay Kit | Chemiluminescent Immunoassay (CLIA) / LC-MS/MS | Quantitative measurement of serum total testosterone; the essential, protocol-dependent first-line test for confirming biochemical hypogonadism (requires AM draw). |
| Luteinizing Hormone (LH) & Follicle-Stimulating Hormone (FSH) Assay Kit | Chemiluminescent Immunoassay (CLIA) | Simultaneous or individual measurement of pituitary gonadotropins to determine etiology (primary vs. secondary hypogonadism) following low testosterone confirmation. |
| Free Testosterone by Equilibrium Dialysis Kit | Equilibrium Dialysis + CLIA / LC-MS/MS | Gold-standard direct measurement of biologically active, unbound testosterone. Critical for accurate assessment in patients with conditions that alter SHBG levels (e.g., obesity, aging, liver disease). |
| Sex Hormone-Binding Globulin (SHBG) Assay Kit | Immunoassay (CLIA) | Measurement of SHBG levels to enable calculation of free and bioavailable testosterone indices, and to interpret total testosterone values in complex clinical contexts. |
| Prolactin Immunoassay Kit | Chemiluminescent Immunoassay (CLIA) | Quantitative measurement of serum prolactin to investigate secondary hypogonadism and rule out prolactinoma as a treatable underlying cause. |
| Estradiol (E2) Sensitive Assay Kit | Chemiluminescent Immunoassay (CLIA) / LC-MS/MS | Sensitive quantification of serum estradiol, important for evaluating hormonal balance, particularly in cases of gynecomastia, obesity, or during testosterone therapy. |
| Iron Status Profile (Ferritin, Transferrin Saturation) | Immunoturbidimetry / Colorimetric | Screening for hereditary hemochromatosis, a treatable cause of primary hypogonadism, through assessment of iron overload. |
| Automated Hypogonadism Diagnostic Panel | Integrated CLIA Platform | A predefined profile for efficient testing, potentially including Total T, LH, FSH, Prolactin, and SHBG to streamline the initial diagnostic workup. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |