- Home
- Resource
- Disease Diagnosis
- Cancers
- Precision in Diagnosis: Advanced Biomarkers and Imaging for Glioblastoma
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Glioblastoma (GBM) is the most aggressive and prevalent primary malignant brain tumor in adults, characterized by its rapid growth and poor prognosis. This resource offers a comprehensive guide to its contemporary diagnostic workup, detailing the integrated clinical pathway from initial symptom assessment and advanced neuroimaging to definitive histopathological confirmation. It further explores the critical role of molecular biomarkers in prognostic stratification.
Glioblastoma (GBM) is the most common and aggressive primary malignant brain tumor in adults, characterized by rapid growth and diffuse infiltration into surrounding brain tissue. Arising from glial cells, it is classified as a Grade IV astrocytoma by the World Health Organization (WHO). Diagnosis typically follows the onset of symptoms like headaches, seizures, or neurological deficits, and is confirmed through neuroimaging and definitive histopathological analysis of tissue obtained via surgery. Despite multimodal treatment involving maximal safe resection, radiation, and chemotherapy, the prognosis remains poor, underscoring the critical need for advanced diagnostic and therapeutic strategies.
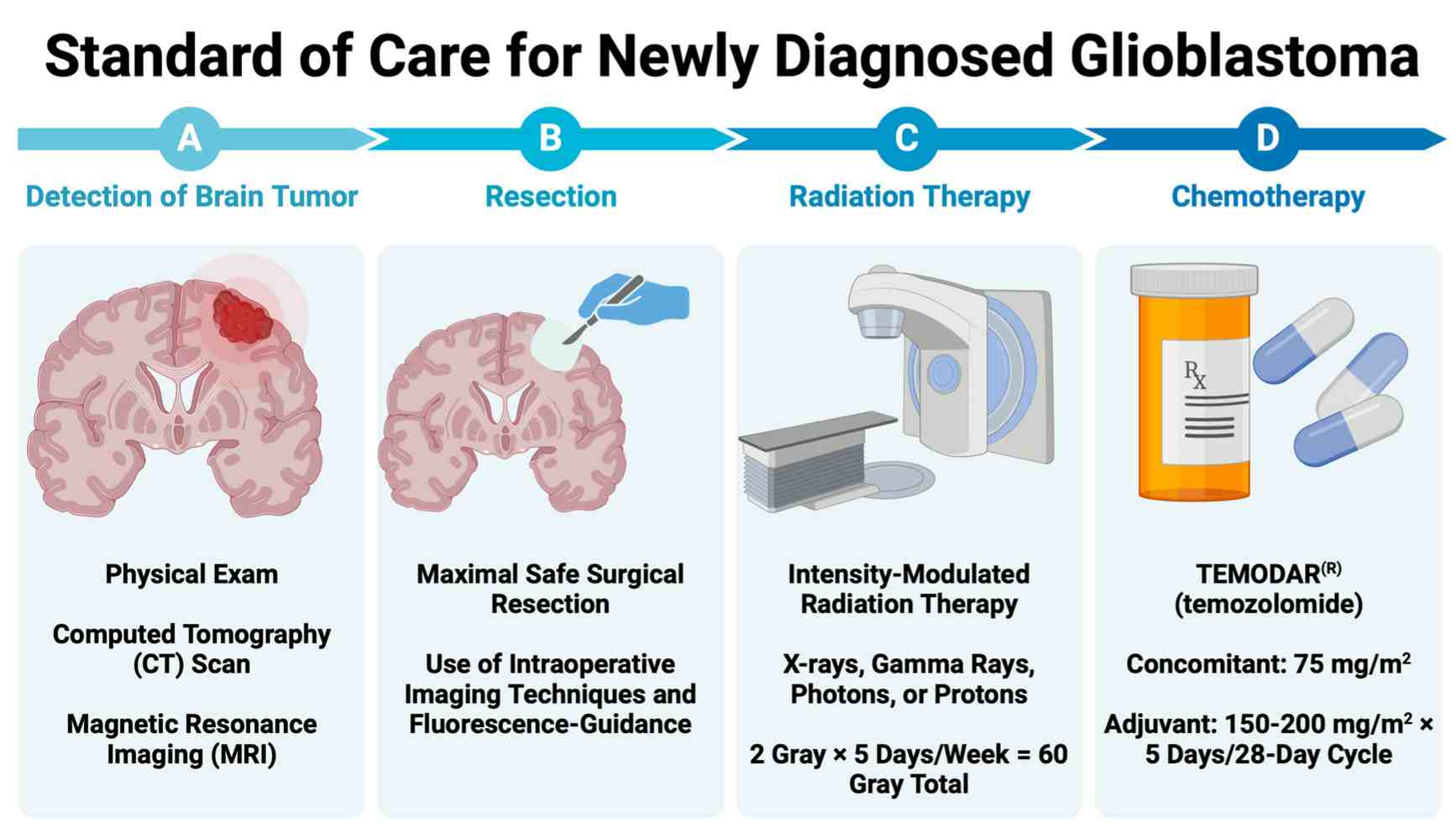 Fig.1 Standard of care for newly diagnosed glioblastoma (GBM). (Rodgers L T, et al., 2024)
Fig.1 Standard of care for newly diagnosed glioblastoma (GBM). (Rodgers L T, et al., 2024)
The diagnosis of glioblastoma (GBM) is often initiated by a constellation of neurological symptoms that prompt a critical investigation using advanced neuroimaging. This process seamlessly connects the patient's clinical presentation with objective radiological findings, where magnetic resonance imaging (MRI) serves as the indispensable tool for characterizing the tumor's location, size, and defining features, guiding the subsequent steps toward a definitive pathological diagnosis.

The clinical presentation of GBM is highly dependent on the tumor's location and size within the brain. Common manifestations include persistent headaches, new-onset seizures, memory or personality changes, and focal neurological deficits such as weakness, sensory loss, or speech difficulties. These symptoms result from a combination of mass effect, infiltration of functional brain tissue, and increased intracranial pressure.

MRI is the cornerstone of radiological evaluation for GBM. A standard protocol with contrast enhancement typically reveals a characteristically heterogeneous ring-enhancing mass surrounded by significant vasogenic edema. Key sequences like T1-weighted post-contrast, T2-weighted, and FLAIR are essential for defining the extent of the lesion, identifying central necrosis, and distinguishing the enhancing tumor from the surrounding infiltrated brain parenchyma.
The definitive diagnosis of glioblastoma (GBM) relies on the direct analysis of tumor tissue, a process that bridges surgical intervention with laboratory pathology. This critical phase begins with a neurosurgeon obtaining a tissue sample, which is then meticulously examined by a pathologist to confirm the malignancy and establish its histological identity, forming the bedrock for all subsequent treatment decisions.

Surgical Resection or Biopsy
The primary method for obtaining tissue is through a surgical procedure, with the goal of maximal safe resection to debulk the tumor and relieve mass effect, while a stereotactic biopsy is performed when the tumor is in an inoperable or eloquent region of the brain. This initial step is crucial, as the collected tissue specimen provides the essential material not only for a histopathological diagnosis but also for comprehensive molecular biomarker testing.
Core Histopathological Features
Under microscopic examination, glioblastoma is characterized by specific hallmarks that distinguish it from other brain tumors. These classic features include significant cellular atypia (abnormal cell appearance), high mitotic activity indicating rapid proliferation, microvascular hyperplasia (the proliferation of small blood vessels), and palisading necrosis, where tumor cells cluster around areas of dead tissue. The identification of these features is fundamental to confirming the World Health Organization (WHO) Grade IV diagnosis.
The landscape of glioblastoma (GBM) diagnostics is rapidly evolving beyond established markers like MGMT and IDH1, with several emerging biomarkers holding promise for revolutionizing patient management. These novel indicators aim to provide a more dynamic, comprehensive, and minimally invasive window into tumor biology, enabling earlier detection, refined prognosis, and real-time monitoring of treatment response and resistance.

In the pursuit of clarity against glioblastoma, Alta DiagnoTech stands as a dedicated partner. Our focused neuro-oncology diagnostics portfolio provides a critical bridge between complex tumor biology and clinical action. By generating precise, actionable data for molecular subtyping, prognostic evaluation, and therapy monitoring, we empower healthcare professionals to make evidence-driven decisions tailored to each patient's disease. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| MGMT Promoter Methylation Assay | PCR / Methylation-Specific PCR | Predictive biomarker analysis for temozolomide chemotherapy response. |
| IDH1/IDH2 Mutation Detection Assay | Next-Generation Sequencing (NGS) / IHC | Diagnostic and prognostic stratification of glioma subtypes. |
| EGFRvIII Mutation Detection Assay | RT-PCR / Digital PCR | Identification of a key therapeutic target and prognostic marker. |
| TERT Promoter Mutation Assay | Sanger Sequencing / NGS | Aid in diagnostic confirmation and prognostic assessment. |
| 1p/19q Co-deletion Status Assay | FISH (Fluorescence In Situ Hybridization) | Differential diagnosis to rule out oligodendroglioma. |
| Liquid Biopsy ctDNA (CSF) GBM Panel | Next-Generation Sequencing (NGS) | Minimally invasive monitoring of tumor genetics and treatment response. |
| Comprehensive GBM Methylation Profiling Panel | DNA Methylation Microarray | Advanced molecular classification for precise tumor subtyping. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |