- Home
- Resource
- Disease Diagnosis
- Cancers
- Precision Diagnostics in Colorectal Cancer: A Guide to IHC and Molecular Biomarkers
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Colorectal cancer (CRC) is a prevalent malignancy whose effective management is critically dependent on precise and comprehensive diagnostic profiling. This resource provides an in-depth guide to the essential in vitro diagnostic (IVD) tools and biomarkers, from immunohistochemistry for mismatch repair proteins to molecular analysis of RAS and BRAF mutations, that are fundamental for accurate diagnosis, prognostication, and guiding targeted treatment decisions in modern clinical practice.
Colorectal cancer (CRC) is a malignant neoplasm arising from the inner lining of the colon or rectum, typically developing from precancerous adenomatous polyps over several years. As one of the most commonly diagnosed cancers worldwide, it represents a major global health challenge. The disease is characterized by a multifactorial etiology, involving genetic predisposition, lifestyle factors, and advancing age. Modern management and improved survival rates heavily rely on early detection through screening and a comprehensive diagnostic approach that integrates histopathological examination with sophisticated biomarker analysis to guide personalized treatment strategies.
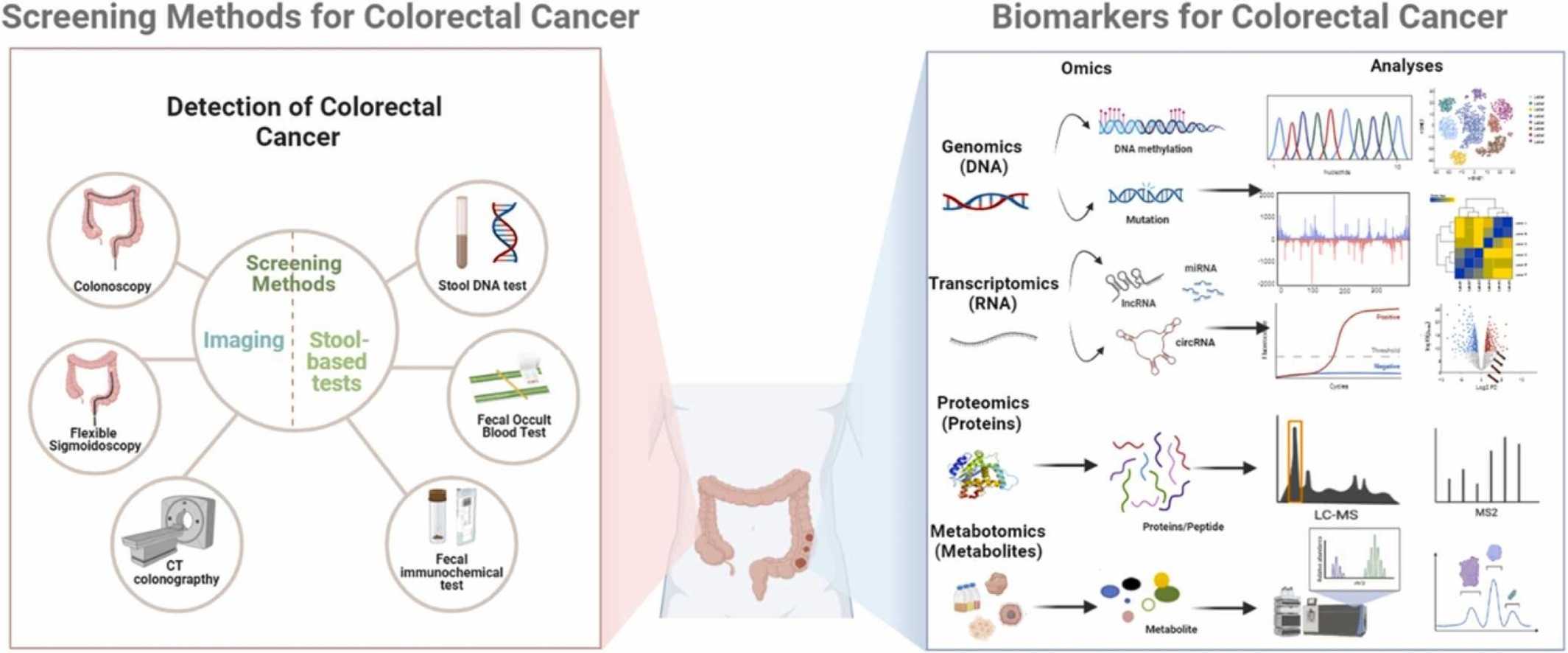 Fig.1 Methods and biomarkers for colorectal cancer (CRC) diagnosis. (Zhang, Yue, et al., 2023)
Fig.1 Methods and biomarkers for colorectal cancer (CRC) diagnosis. (Zhang, Yue, et al., 2023)
Histopathology remains the undisputed cornerstone for the definitive diagnosis of colorectal cancer (CRC). It involves the microscopic examination of tissue samples—obtained via biopsy or surgical resection—to visualize the cellular abnormalities that define malignancy. This process transforms a clinical suspicion into an objective diagnosis, providing the essential foundation upon which all subsequent treatment decisions are built. The pathologist's initial assessment using routine hematoxylin and eosin (H&E) staining confirms the presence of cancer, classifies the tumor type (overwhelmingly adenocarcinoma), and evaluates the tumor grade by assessing the degree of gland formation and cellular differentiation.
Furthermore, on surgical specimens, histopathology is critical for accurate pathological staging, determining key prognostic factors such as the depth of tumor invasion (T stage), the presence of lymph node metastases (N stage), and the status of critical resection margins, most notably the circumferential resection margin in rectal cancer. In essence, histopathology provides the initial, indispensable "what and where" of the cancer, setting the stage for more refined biomarker testing.
While histopathology provides the initial diagnosis, immunohistochemistry (IHC) serves as a powerful and indispensable ancillary tool that resolves diagnostic challenges and unlocks critical prognostic and predictive information. By detecting specific antigenic markers within tumor tissue, IHC provides an objective, biomarker-driven layer of analysis that guides precision medicine in colorectal cancer (CRC).
Screening for Lynch Syndrome
Immunohistochemistry (IHC) serves as a primary screening tool for Lynch syndrome by detecting the loss of nuclear expression in the four core mismatch repair (MMR) proteins: MLH1, MSH2, MSH6, and PMS2. A distinctive pattern of loss (e.g., absent MSH2/MSH6) provides a powerful indicator of this hereditary condition, effectively guiding subsequent genetic testing. This critical application enables the identification of patients and families at high risk, facilitating targeted surveillance and preventive strategies.

Differentiating Colorectal Cancer from Other Malignancies
IHC is essential for determining the primary origin of metastatic or poorly differentiated carcinomas. A targeted antibody panel can confirm a colorectal origin, typically demonstrating a profile positive for CK20, CDX2, and SATB2, while often negative for CK7. This differentiation is crucial for accurate diagnosis and appropriate treatment planning, especially when a metastasis is the initial presentation of the disease.

Resolving Diagnostic Challenges in Poorly Differentiated Tumors
When routine histology is ambiguous, IHC provides definitive clarity to distinguish poorly differentiated colorectal adenocarcinoma from other malignancies with similar morphology. By using specific markers—such as cytokeratins to confirm carcinoma, CD45 to rule out lymphoma, or neuroendocrine markers (e.g., synaptophysin) to identify neuroendocrine tumors—IHC resolves these diagnostic dilemmas with high accuracy.
Prognostic and Predictive Biomarker Analysis
Beyond diagnosis, IHC directly informs patient prognosis and therapy selection. The assessment of MMR protein status (dMMR) not only screens for Lynch syndrome but also predicts a high likelihood of response to immunotherapy. Furthermore, IHC for the BRAF V600E mutant protein can rapidly identify tumors with a poor prognostic profile, offering vital information for risk stratification and treatment decisions.
Molecular biomarker testing has become a standard and indispensable component of the diagnostic workup for colorectal cancer (CRC), moving beyond morphology to guide personalized treatment strategies and provide critical prognostic information. The analysis of specific genetic alterations in tumor tissue enables a precision medicine approach, ensuring patients receive the most effective therapies while avoiding those unlikely to be beneficial.
Testing for mutations in the KRAS and NRAS genes is a critical predictive biomarker for metastatic colorectal cancer. Patients whose tumors harbor a mutation in these genes are highly unlikely to respond to anti-EGFR monoclonal antibody therapies, such as cetuximab and panitumumab. Therefore, RAS genotyping is mandatory to identify the subset of patients with wild-type tumors who are eligible for this targeted treatment, ensuring therapy is directed to those most likely to benefit.

The BRAF V600E mutation serves as a key prognostic and predictive biomarker, identifying a distinct and aggressive molecular subtype of colorectal cancer. Its presence is associated with significantly poorer survival outcomes and often occurs in sporadic tumors characterized by mismatch repair deficiency. Beyond prognosis, detecting this mutation is increasingly relevant as it can guide the use of specific targeted therapy combinations in the metastatic setting.

Analysis of MMR/MSI status is essential for both identifying Lynch syndrome and, more broadly, as a predictive biomarker for immunotherapy. Tumors classified as MMR-deficient (dMMR) or MSI-High have a high mutational burden, which makes them exceptionally responsive to immune checkpoint inhibitors. This classification represents a major treatment advancement, fundamentally altering the therapeutic landscape for this specific patient subgroup.
Alta DiagnoTech is dedicated to advancing the precision diagnosis of colorectal cancer (CRC) by providing a comprehensive portfolio of high-quality in vitro diagnostic (IVD) products. From robust immunohistochemistry (IHC) assays to sophisticated molecular tests, our product portfolio is designed to support the entire diagnostic workflow, ensuring that every patient receives a precise and actionable diagnosis. If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| MMR Status IHC Panel | Immunohistochemistry (IHC) | Simultaneous detection of MLH1, MSH2, MSH6, and PMS2 protein loss for Lynch syndrome screening and identification of dMMR tumors eligible for immunotherapy. |
| BRAF V600E IHC Assay | Immunohistochemistry (IHC) | Rapid and highly specific detection of the BRAF V600E mutant protein to identify patients with a poor-prognosis CRC subtype. |
| CRC Differentiation IHC Panel | Immunohistochemistry (IHC) | Aids in determining the origin of carcinomas using markers like CK20, CDX2, and SATB2 to confirm a colorectal primary. |
| KRAS/NRAS Mutation Detection Kit | Real-Time PCR | Identifies specific mutations in the KRAS and NRAS genes to predict non-response to anti-EGFR therapy in metastatic CRC patients. |
| BRAF Mutation Detection Kit | Real-Time PCR | Accurately detects the BRAF V600E mutation and other variants to provide comprehensive prognostic information. |
| MSI Analysis System | Fragment Analysis (PCR) | Molecular determination of Microsatellite Instability (MSI) status to confirm dMMR and predict response to immunotherapy. |
| Next-Gen CRC Focus Panel | Next-Generation Sequencing (NGS) | A comprehensive panel for the simultaneous analysis of mutations in KRAS, NRAS, BRAF, and other key genes, plus MSI status, from a single sample. |
References
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |