- Home
- Resource
- Disease Diagnosis
- Cancers
- Precision Diagnosis of Endometrial Cancer: Integrating Biomarkers and Modern IVD into Clinical Practice
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Endometrial cancer is the most prevalent gynecologic malignancy in the developed world, and its diagnosis has been transformed by the integration of molecular classification. This resource provides a comprehensive guide to the modern diagnostic pathway for endometrial cancer, detailing how in vitro diagnostic (IVD) technologies are essential for precise patient stratification. We will explore the critical role of key biomarkers and how they integrate into clinical practice to guide prognosis and personalized treatment decisions.
Endometrial cancer, a malignancy arising from the inner lining of the uterus, stands as the most common gynecologic cancer in the developed world. While often diagnosed at an early stage due to postmenopausal bleeding as a presenting symptom, its clinical behavior and patient outcomes can vary significantly. Historically, classification relied solely on histology; however, the integration of molecular subtyping has revolutionized its diagnosis. Modern pathology now leverages key biomarkers to move beyond a one-size-fits-all approach. This shift towards precision medicine is critical for accurately determining prognosis and guiding personalized, effective treatment strategies for patients.
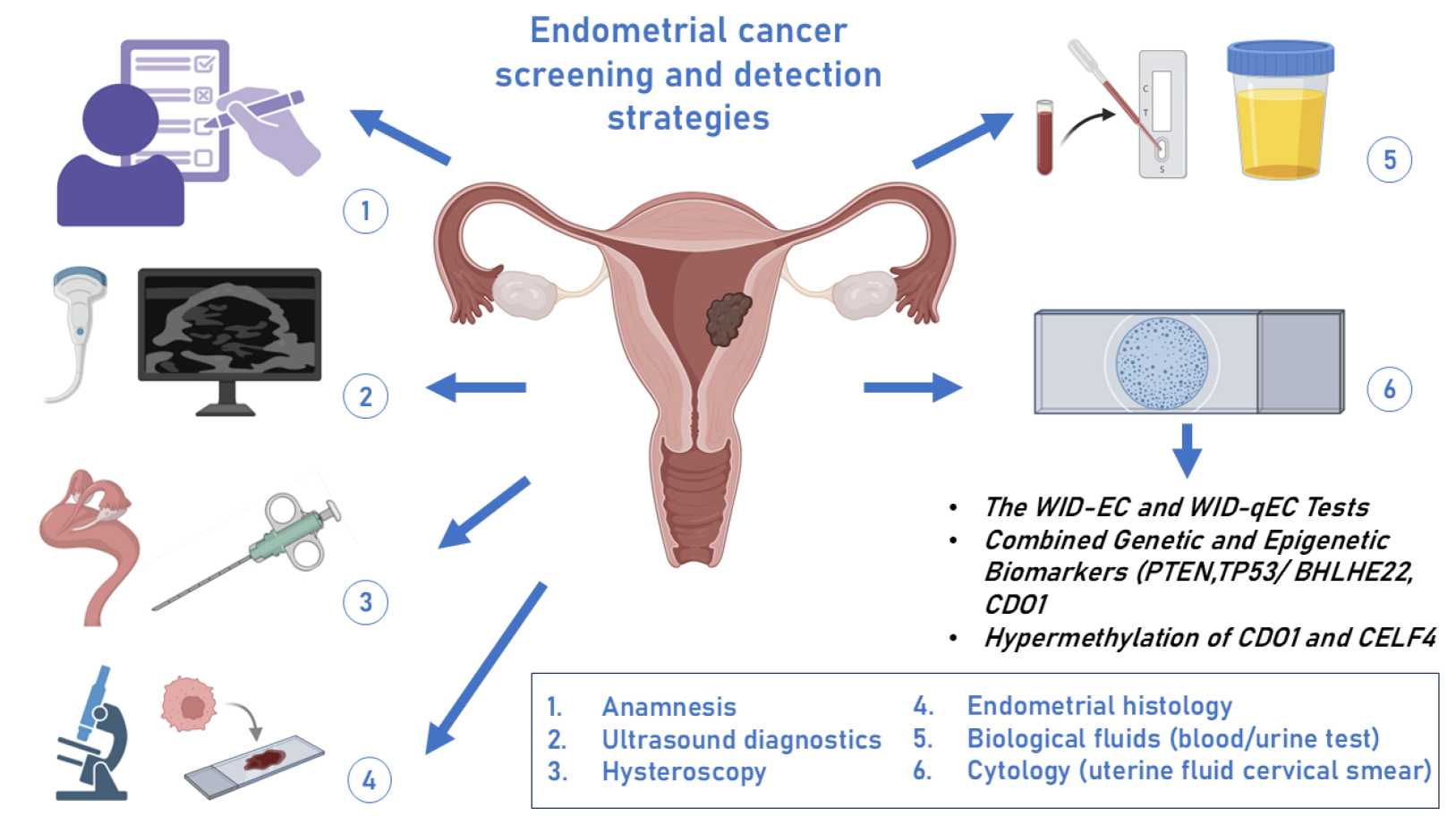 Fig.1 Current approaches to the detection and screening of endometrial cancer. (Asaturova A, et al., 2024)
Fig.1 Current approaches to the detection and screening of endometrial cancer. (Asaturova A, et al., 2024)
The definitive diagnosis of endometrial cancer rests upon a cornerstone of histopathologic examination, making the procurement of a representative tissue sample the critical first step in the diagnostic cascade. This initial workup moves from suspicion based on clinical presentation, most commonly abnormal uterine bleeding, to concrete pathological confirmation, a process that also utilizes imaging to provide crucial contextual information about the uterus and pelvis. The following two components are fundamental to establishing a baseline diagnosis and guiding subsequent management.
The modern diagnosis of endometrial cancer is defined by the integration of specific biomarkers that move beyond histology to reveal a tumor's molecular identity. This paradigm shift, driven by The Cancer Genome Atlas (TCGA) classification, is enabled by advanced in vitro diagnostic (IVD) technologies. These "pillars of precision" provide critical prognostic information and guide targeted treatment decisions, forming the core of contemporary patient management. The key biomarkers and their associated IVD methodologies are outlined below.
This framework categorizes endometrial cancer into four distinct molecular subtypes, each with specific prognostic implications and detected through a combination of IVD tests.
| Molecular Subtype | IVD Technology | Clinical Value |
| POLE Ultramutated | Next-generation sequencing (NGS) to identify pathogenic mutations in the POLE exonuclease domain. | Identifies patients with an excellent prognosis, who may be candidates for treatment de-escalation. |
| MSI-High (Microsatellite Instability-High) |
|
Prognostic stratification and a predictive biomarker for immunotherapy. |
| p53 Abnormality | IHC (aberrant overexpression/absence) and/or NGS (TP53 mutation sequencing). | Identifies clinically aggressive tumors with a poor prognosis, guiding more intensive therapy. |
| No Specific Molecular Profile (NSMP) | A diagnosis of exclusion when the other three classifiers are negative. | Intermediate prognosis; risk is further defined by traditional histologic factors. |
Serum CA-125
While not useful for screening or early diagnosis due to low specificity, the serum biomarker CA-125 is a valuable monitoring tool in established endometrial cancer. It is primarily measured via immunoassay and is most clinically relevant for tracking disease burden and treatment response in patients with advanced-stage or recurrent disease, as elevated levels often correlate with extra-uterine spread.
Hormone Receptor Status (ER/PR)
The status of estrogen (ER) and progesterone receptors (PR), determined by immunohistochemistry (IHC) on tumor tissue, provides critical prognostic information. Tumors that are ER/PR positive are typically associated with a more favorable outcome. Furthermore, this biomarker is predictive, as it helps identify patients who may be candidates for hormonal therapies, especially in the setting of advanced or recurrent disease.

The modern clinical diagnostic pathway for endometrial cancer strategically integrates IVD testing to transform a standard tissue diagnosis into a precise molecular profile for personalized management. This process typically begins with a universal triage step using immunohistochemistry (IHC) for MMR proteins and p53 on all tumor samples, which efficiently identifies most molecular subtypes. Cases with specific findings, such as MMR deficiency or ambiguous histology, then trigger reflexive testing, such as MLH1 methylation analysis or next-generation sequencing (NGS) for POLE and TP53. This structured, algorithmic approach ensures that critical biomarker data is seamlessly generated from the initial biopsy, directly informing prognostic stratification and guiding decisions regarding adjuvant therapy, surgical intensity, and eligibility for targeted treatments like immunotherapy.
Alta DiagnoTech provides a comprehensive portfolio of IVD solutions for endometrial cancer, enabling laboratories to deliver a complete and precise molecular diagnosis. Our products are designed to seamlessly integrate into the modern diagnostic pathway, from initial histology to full molecular subtyping according to the latest TCGA classification.If you have related needs, please feel free to contact us for more information or product support.
| Product Name | Technology | Application |
| MMR IHC Panel | Immunohistochemistry (IHC) | First-line screening for microsatellite instability (MSI) via detection of MMR protein (MLH1, MSH2, MSH6, PMS2) loss. |
| p53 IHC Ready-to-Use | Immunohistochemistry (IHC) | Detection of aberrant p53 protein expression (overexpression or null phenotype) to identify TP53-mutant tumors. |
| ER/PR IHC Panel | Immunohistochemistry (IHC) | Simultaneous assessment of Estrogen and Progesterone Receptor status for prognosis and therapy guidance. |
| Endometrial NGS Panel | Next-Generation Sequencing (NGS) | Comprehensive profiling for POLE mutations, MSI status, TP53 mutations, and other relevant genomic alterations. |
| MSI Direct PCR Kit | Polymerase Chain Reaction (PCR) | Definitive, high-sensitivity determination of Microsatellite Instability status. |
| MLH1 Methylation Assay | Methylation-Specific PCR | Differentiation between sporadic (methylated) and Lynch syndrome-associated (unmethylated) MLH1 deficiency. |
| CA-125 Immunoassay | Electrochemiluminescence Immunoassay (ECLIA) | Quantitative measurement of serum CA-125 levels for monitoring advanced or recurrent disease. |
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |